Hopes Dim for Struggling Field
By Richard Jefferys
Immunizations in a trial of Merck’s HIV vaccine were stopped when it was determined that the vaccine was not working. There are now concerns that the vaccine may have increased the risk of HIV infection in some participants.
On September 18, 2007, the world of HIV vaccine research was dealt a dismaying and unanticipated blow: immunizations in an ongoing efficacy trial of Merck’s HIV vaccine candidate were stopped when the Data Safety Monitoring Board (DSMB) conducted a planned interim analysis of the results and concluded that the vaccine was ineffective, both at preventing HIV infection and reducing viral loads in immunized individuals who became HIV infected. Results from the trial were not anticipated until 2009 and, while there was certainly some skepticism about whether the approach would work, nobody predicted that the vaccine would fail so quickly and unequivocally.
After the DSMB’s decision was announced publicly, concern began to mount that the vaccine had not only failed to work but also somehow increased susceptibility to HIV infection among a subset of the trial participants. The basis for this concern was finally revealed when the trial data were presented at a meeting of the HIV Vaccine Trials Network (HVTN) in Seattle on November 7. The results indicate that vaccine recipients with high levels of antibodies against the virus used as a vector in the Merck trial—a weakened form of a cold-causing virus called adenovirus serotype 5 (Ad5, for short)—were more likely to acquire HIV infection than those who got a placebo shot. Ad5 vectors are being used in a number of vaccine studies for diseases such as malaria, TB, and Ebola, and the next large HIV vaccine efficacy trial is slated to also include an Ad5- based vaccine as a booster shot following a series of DNA immunizations. The Merck results have caused all Ad5 vaccine trials to be placed on hold while researchers attempt to figure out what happened.
To follow the unfolding story of the Merck trial—a collaborative effort between Merck and the HVTN called STEP or HVTN 502—it’s important to know the background to both the vaccine construct and the design of the clinical trial. Merck initially made a prototype Ad5 vaccine that expressed a single protein (Gag) from HIV-1 subtype B and used this version to conduct preliminary studies. These studies showed that the vaccine triggered the development of CD4 and CD8 T-cell responses against the HIV Gag protein in the majority (~60–70%) of recipients; this was something of a breakthrough as previous vaccine candidates had induced CD8 T-cell responses in only around a third of recipients (at best). However, the studies also showed that the Merck vaccine was far less effective at inducing Gag-specific T-cell responses in people who had been exposed to the natural form of Ad5 and had high levels of anti-Ad5 antibodies.
As a result of these initial data, Merck and the HVTN designed a “test of concept” efficacy trial, STEP/HVTN 502. The idea was to try and test whether the HIV-specific T-cell responses induced by the vaccine could offer benefit—either in terms of preventing infection or reducing viral loads—to individuals at a high risk of acquiring HIV infection. But because idea was to test the efficacy of vaccine-induced HIV-specific T-cell responses, a decision was made to limit the trial to individuals who responded best to the vaccine: those with low levels of antibodies against Ad5 (defined as an antibody titer <1:200). The target sample size for the trial was 1,500 and it began enrolling in December of 2004. To increase the number of parts of HIV being targeted, the final version of the Merck Ad5 vaccine used in the trial was a “trivalent” mixture of three Ad5 vectors, one that encoded Gag and two additional vectors encoding the HIV-1 Nef and Pol proteins.
Shortly after STEP began enrolling, new data from phase I studies of the trivalent version of the Merck Ad5 vaccine suggested—for reasons that are still unclear—that it was less affected by the presence of anti-Ad5 antibodies than the prototype Gag-only vaccine. In other words, even among people with relatively high levels of anti-Ad5 antibodies, the majority of recipients developed T-cell responses to the three HIV proteins produced by the vaccine. What turned out to be a fateful decision was made: to enroll another 1,500 people in STEP without regard to their anti-Ad5 antibody titer. Enrollment of this second cohort began around September of 2005. Participants in the trial included men and women aged 18–45 at risk for HIV infection due to sexual activity; injection drug users were not excluded but needed to have additional sexual risk factors. Study sites were located in the US, Puerto Rico, Canada, Haiti, Brazil, Peru, Jamaica, the Dominican Republic, and Australia. Overall, STEP had two goals, called coprimary endpoints: to assess whether (1) the vaccine could prevent HIV infection and/or (2) reduce viral load in vaccine recipients who became infected during the trial. Interim analyses of the data by the DSMB were built into the study design; the trigger for the first such analysis was the occurrence of 30 HIV infections in the initial low anti- Ad5 antibody titer cohort.
It was reaching this trigger point that prompted a meeting of the DSMB on September 18. The DSMB reviewed data from the first 1,500-person cohort enrolled in the trial and found that of the 45 infections that had occurred, 24 were in the vaccine group and 21 in the placebo (dummy vaccine) group. Among these infected participants, viral load levels measured 8–12 weeks after infection were similar; approximately 40,000 copies in the vaccine group and 37,000 copies in the placebo group. Additionally, there was a worrying difference in the number of infections between vaccine and placebo recipients when the analysis was restricted to individuals who had received at least two shots of either vaccine or placebo; in this subgroup, there were 19 infections in the vaccine group compared to 11 in the placebo group. In line with the original study design, the DSMB stopped further immunizations in the trial because of what researchers call “futility”—even if the study were to continue, there was no possibility of the vaccine showing any efficacy.
The news provoked widespread disappointment. Although there was very little evidence to suggest that vaccine-induced T-cell responses could fully protect against HIV infection, data from animal studies offered reason to hope that the vaccine might reduce viral loads in study participants who became infected. This hope was clearly not borne out. But worse news was to come. Another recently initiated HVTN trial in South Africa of the same Merck Ad5 vaccine—the Phambili/HVTN 503 trial—was placed on hold due to the STEP results. When the Phambili DSMB subsequently reviewed the STEP data, they not only permanently halted the Phambili trial but also recommended counseling participants that the vaccine might have enhanced susceptibility to HIV infection.
Table 1: STEP Trial Results – Number of Infections and Post-Infection Viral Load Levels
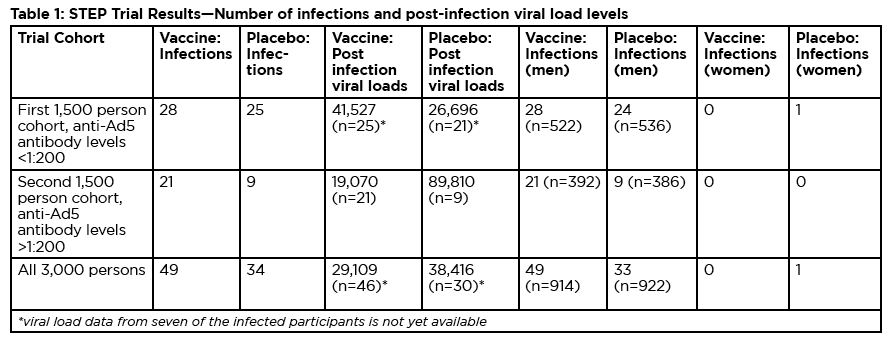
The basis for these recommendations became clear on November 7 at the HVTN meeting. In the second 1,500-person STEP cohort (involving individuals with anti-Ad5 antibody titers > 1:200), the skewing of infections between vaccine and placebo groups was even more notable: there 21 infections among vaccine recipients and 9 in the placebo group. Taking into account an additional eight infections in the initial cohort that occurred after the September 18 DSMB review (evenly split between vaccine and placebo groups), the totals became 49 infections in the vaccine group and 34 in the placebo group. The infections were almost entirely among the 1,825 male trial participants; only one of the 1,185 women in the study became infected—in the placebo group.
Due to the relatively small size of the study and the fact that subanalyses of the results among the second STEP cohort and men versus women were not planned in advance, the difference in infections between the vaccine and placebo groups does not quite attain statistical significance. But there is a complete consensus among the researchers involved that it is an extremely strong trend that demands investigation and explanation. Adding to the concern is an apparent association with baseline levels of anti- Ad5 antibody levels: the higher the baseline titer, the greater the imbalance of infections between the vaccine and placebo groups (see table 2).
| Table 2: Number of Infections Dependent on Baseline Anti-Ad5 Antibody Titers | ||
| Anti-Ad5 antibody titer | Infections, Vaccine | Infections, Placebo |
| less than 1:18 | 20 | 20 |
| 1:18-1:200 | 8 | 4 |
| 1:200-1:1000 | 14 | 7 |
| over 1:1000 | 7 | 2 |
| Table 3: HIV Incidence Declined with Increased Anti-Ad5 Antibody Titers in the Placebo Group | |||
| Anti-Ad5 Antibody Titer | HIV Incidence Vaccine (95% CI) | HIV Incidence Placebo (95% CI) | Relative Incidence (Vaccine:Placebo) |
| less than 1:18 | 4% (2.5–6.3) | 4% (2.5–6.2) | 1.0 |
| 1:18–1:200 | 4.4% (1.9–8.8) | 2.2% (0.6–5.5) | 2.0 |
| 1:200–1:1000 | 6.1% (3.3–10.2) | 3% (1.2–6.2) | 2.0 |
| over 1:1000 | 4.4% (1.8–9.1) | 1.2% (0.2–4.5) | 3.0 |
|
Adenovirus-Specific T-Cell Immunity |
| One of the yawning information gaps highlighted by the Merck HIV vaccine trial is the absence of data regarding the impact of vaccination on adenovirus-specific T-cell immune responses. Although it was logical for researchers to focus on the HIV-specific T-cell responses induced by the vaccine, in retrospect it was an oversight to not pay attention to the effect of the vector on adenovirus-specific T cells-particularly CD 4 T cells, which are potential targets for HIV infection.
The literature on adenovirus-specific T-cell immunity is relatively sparse, but the published data indicates: Adenovirus-specific T-cell responses are detectable in the majority of individuals studied. Responses are biased toward CD 4 T cells with an effector memory phenotype. Adenovirus-specific CD 4 T-cell responses are generally of a high magnitude but wane with age. Adenovirus-specific CD 4 T cells recognize epitopes that are conserved across adenovirus serotypes, including epitopes that are present even in Ad5 vectors with multiple gene deletions. Taken together, the published data certainly suggests that studies of the impact of Merck’s Ad5 vector on adenovirus-specific CD 4 T cells should be a priority in the ongoing effort to understand the outcome of the Merck HIV vaccine trial. |
It seems very possible that, in STEP, the levels of anti-Ad5 antibodies also correlated with susceptibility to HIV infection; this would explain why placebo recipients with high anti-Ad5 titers had a lower incidence of HIV infection. If true, however, there is still a need to explain why receipt of the Merck vaccine appeared to override the reduced susceptibility to HIV infection associated with high anti-Ad5 titers.
Buchbinder’s initial evaluation of study participants uncovered some significant differences between the first and second STEP cohorts, but no indications of any important differences between vaccine and placebo recipients in either cohort. Buchbinder emphasized that because of the small size of the study and the concentration of the infections among an even smaller subset of the overall population (the 1,825 men) it remains possible that the results reflect the play of chance. But given the serious implications if the Ad5 vector did enhance susceptibility in people with high anti-Ad5 antibody levels, Buchbinder argued that chance must be considered as an explanation only when all other potential factors have been evaluated and ruled out.
Juliana McElrath from the HVTN and Danny Casimiro from Merck are taking the lead on evaluating biological mechanisms that could account for the STEP results. At the Seattle meeting, McElrath reviewed the current status of these efforts. In terms of why the vaccine failed, McElrath and colleagues are looking particularly at the breadth and functional capabilities of the vaccine induced HIV-specific T-cell responses. Results to date indicate that, on average, vaccine recipients developed CD8 T-cell responses to just one epitope from each HIV protein in the vaccine (Gag, Pol, and Nef). Preliminary data indicates that the responses were functional but additional analyses are being conducted to look at a number of potentially important features of the HIV-specific T cells, including their phenotype (effector memory vs. central memory, two slightly different types of memory T cells), ability to proliferate (copy themselves), ability to produce multiple cytokines and chemokines (sometimes referred to as polyfunctionality) and ability to kill HIV-infected cells in vitro (in a lab dish).
Shifting to the evidence of enhanced susceptibility, McElrath outlined the leading hypotheses that might explain the data: generalized immune activation as a result of immunization, immune responses to the Ad5 vector, and/or immune responses to the HIV proteins produced by the vaccine. McElrath also highlighted the importance of studying whether repeated doses of the vaccine impacted the outcome, since the Ad5 vector itself would have led to the development of anti-Ad5 antibodies in individuals who had low titers at baseline. McElrath showed data indicating that Ad5 immunization does increase immune activation as measured by levels of the cytokines IL-6, IL-10, TNF-alpha, and IP-10, but levels return to baseline by seven days after immunization. Further studies are being performed, but the presence of anti-Ad5 antibodies would be expected to reduce this immune-activating effect rather than enhance it. Another key question is whether the vaccine boosted the number of potential target cells for HIV by increasing the numbers of activated, CCR5-expressing CD4 T cells (particularly Ad-specific CD4 T cells, which would be stimulated by the vaccine). McElrath reported that individuals with anti-Ad5 antibody titers above 1:200 did have significantly more activated, CCR5-expressing CD4 T cells but there were no apparent differences between vaccine and placebo recipients at week 30 of the trial (four weeks after the last immunization). More detailed analyses involving additional time-points are underway. McElrath listed other priority areas for future studies:
Defining Ad5-specific immune responses (T cells and neutralizing antibodies) and possible association with increased acquisition (Ad5-specific CD4 T-cell responses would be activated by the vaccine, and some researchers have speculated that these Ad5-specific CD4 T cells may have provided additional targets for HIV infection)
- Examining the potential effect of repeated vaccine doses
- Examining the effect of immunization on CD4 T-cell numbers, activation state and CCR5 expression in the rectum and lower genital tract
- Exploring the relationship of specific Ad5 gene deletions with increased CD4+ T cell activation
- Assessing in vitro susceptibility of CD4 T cells, dendritic cells, and macrophages in participants with low versus high anti-Ad5 antibody titers
|
Results among Women |
| The sponsors of STEP made a laudable effort to ensure that at least a third of study participants were women (who represented 38% of the total study population). However, the occurrence of only one infection among women (in the placebo group) leaves questions about sex-specific efficacy—or harmful effects—of the vaccine unanswered. The results suggests that women in the trial were not as frequently exposed to HIV as the researchers had predicted, and/or that behavioral prevention interventions that were part of the study protocol were particularly effective in reducing the risk behaviors of women trial participants. It will be important for researchers and advocates to delve further into the STEP results among women in order to ensure that future trials can adequately answer questions about the sex-specific effects of vaccine candidates. |
An expanded version of the HVTN’s Laboratory Science Committee, chaired by Bruce Walker, will be responsible for developing the complete agenda for follow-up studies pertaining to STEP. The HVTN will also use their website to solicit applications from outside investigators who may be able to contribute to the analysis effort.
After the November 7 HVTN meeting, the decision was made to unblind STEP and inform all participants whether they received vaccine or placebo; participants will also be informed of their baseline anti-Ad5 antibody titer and counseled about the possibility that the vaccine may have enhanced susceptibility to HIV infection in individuals with high titers. Participants will continue to be followed in the hopes of evaluating the long-term effects of the vaccine. So far, of the infections in the study that occurred after week 52, seven were in vaccine recipients and six in placebo, perhaps providing some reason to hope that any enhancing effect—if real—was transient.
The worst-case scenario raised by the STEP results is that the HIV-specific T-cell responses induced by the vaccine were somehow harmful. But if that were the case, then the worst outcomes in the trial would have been among participants with the highest levels of HIV-specific T cells (i.e., individuals with low titers of anti-Ad5 antibodies). The fact that the trend toward enhanced susceptibility was only seen in individuals with anti- Ad5 antibodies suggests that immune responses to the Ad5 vector itself — or some interaction between the vector, vector-specific immunity, and potential target cells for HIV (such as CD4 T cells and dendritic cells) — are more likely culprits.
On December 12, the AIDS Vaccine Research Subcommittee (AVRS) of the National Institute of Allergy and Infectious Disease (NIAID) met to discuss the implications of the STEP trial for the next planned NIAID-sponsored HIV vaccine efficacy trial, dubbed PAVE100. This trial involves two candidates designed by the Vaccine Research Center (VRC) at the National Institutes of Health, a DNA vaccine (given three times) followed by a single shot of an Ad5 vector as a boost. The vaccines contain additional HIV antigens not used in the Merck trial (Env proteins from clades A, B, and C) and the Ad5 vector also has additional genes deleted (the Merck construct is missing a gene called E1 and part of E3; the VRC’s also has the E1, E3, and E4 genes removed) which is intended to reduce the magnitude of the immune response against the Ad5 vector.
However, it remains possible that the VRC’s Ad5 vector could have a similarly detrimental effect on susceptibility to that seen in the Merck trial. One means to reduce this risk—apparently already adopted by PAVE100 proponents—is to limit enrollment to individuals with zero antibody titers against Ad5. However, to do so would be to ignore the evidence that the magnitude of antibody responses is an indicator of the quality of an individuals immune response; in effect, the trial would be limiting enrollment to people with the highest susceptibility to HIV infection because of their qualitatively poorer immune response (in other words, a population least likely to benefit from a vaccine, even if it was efficacious). This presents a catch-22 from which PAVE100 may not be able to escape, because unless a convincing explanation for the STEP results which exonerates Ad5 is eventually forthcoming, it would certainly not be appropriate or ethical to give an Ad5 vector to a representative population of individuals without regard to anti-Ad5 antibody titers, because of the risk of enhancement. Another AVRS meeting and discussion is planned in order to offer a formal recommendations regarding PAVE100, and it will be important for these issues to be explored and discussed in more detail.
TAG will continue to monitor and report on developments as analysis of the STEP data continues.
| Links |
| Data presentations from the HVTN Meeting: www.hvtn.org/science/1107.html
News and commentary from TAG’s Basic Science, Vaccines, and Prevention Project Weblog: http://tagbasicscienceproject.typepad.com Information, links and Q&As from the AIDS Vaccine Advocacy Coalition: www.avac.org/pr_step_study.htm Special bulletins from the IAVI Report: |
