Research and Policy Recommendations for HCV/HIV Coinfection
February 2003
Introduction
The unfolding epidemic of hepatitis C virus (HCV) infection is a serious and growing problem. An estimated 170 million people around the world are infected. In the United States, at least four million people have been exposed to HCV, and 2.7 million of them have developed chronic hepatitis C. Chronically infected persons can either remain asymptomatic, progress very slowly, maintain mild to moderate liver scarring or develop serious liver damage, such as cirrhosis or hepatocellular carcinoma (HCC). Hepatitis C-related liver damage has become the chief cause for liver transplantation in this country, and ten to twelve thousand people die each year from HCV-associated end-stage liver disease (ESLD). HCV disease is a particularly severe problem for HIV-positive people. Up to a quarter of all people with HIV in the U.S. may be coinfected with HCV. The progression of hepatitis C is accelerated in HIV-positive individuals, and HCV-related ESLD has become a leading cause of death in those with HIV.
The current state of research on HCV infection lags far behind that on HIV. For example, it is not yet possible to grow infectious hepatitis C virions in tissue culture, and there is still no adequate animal model for HCV infection or disease. These limits have seriously hampered the understanding of HCV’s replication cycle and have impeded development of new treatments. The best current combination therapy for hepatitis C (pegylated interferon and ribavirin) fails at least half those who undergo treatment, and the range and severity of its side effects can seriously affect patients’ quality of life, adherence, and chances for a successful outcome. Clearly more and better treatments are needed.
Although millions of Americans are infected and at risk for progression to serious disease, there is no federally funded infrastructure to coordinate education, prevention, testing, care, and treatment for HCV infection. The lack of a comprehensive plan to reduce HCV incidence — particularly through increasing access to sterile syringes — means that existing prevention programs have scattered and limited impact. Many individuals lack access to costly HCV treatment, including the underinsured and uninsured, while cash-strapped AIDS Drug Assistance Programs (ADAPs) are in most cases unable to add expensive HCV care to their already overburdened portfolios. Prisoners, among whom hepatitis C is endemic, have had to resort to litigation to obtain treatment. Projections of HCV-related morbidity and mortality in mono- and HIV coinfected individuals forecast a significant upsurge in health care costs, illness, and loss of life over the next twenty years. The time to step up action to address gaps in research and policy is now.
TAG’s first hepatitis report, by Michael Marco and Jeffrey Schouten, released in July of 2000, was written to provide affected individuals, clinicians, researchers, educators and policy makers a detailed overview of hepatitis C and HIV/HCV coinfection. The report concluded with a set of research and policy recommendations (see Appendix A). In the spring of 2003, TAG will publish a new version of The Hepatitis C/HIV Coinfection Report that will include a revised and expanded set of research and policy recommendations for HCV and HIV/HCV coinfection research, prevention and care programs. These draft recommendations have been developed following a comprehensive literature review of more than 500 journal articles, abstracts from conferences on HCV and HIV/HCV, updated treatment guidelines, and interviews with HCV and HIV/HCV coinfected individuals, researchers, physicians, harm reduction experts, health educators, public health officials, and activists and advocates from both the HCV and HIV communities.
The growing voice of HCV advocacy and the increasing attention given to HCV coinfection by HIV activists suggest that the timing is right for a broad-based coalition to press for a comprehensive research agenda and increased access to treatment. With these recommendations, TAG is hoping to broaden dialogue and collaboration among activists, policy makers, researchers, funders, educators and, especially, people with HCV and HIV/HCV coinfection.
Executive Summary: Research and Policy Recommendations
TAG’s recommendations fall into six categories:
- Epidemiology, Transmission and Prevention;
- Pathogenesis and Natural History;
- Diagnostics;
- Care and Treatment;
- Key Treatment Questions in HIV/HCV Coinfection; and
- Basic Research and Drug Development.
1. Epidemiology, Transmission and Prevention
1a. Implement national surveillance for chronic hepatitis C infection.
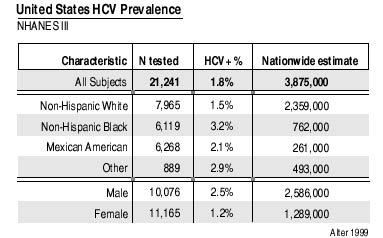
Hepatitis C virus (HCV) was identified in 1988, and the development of an antibody test soon followed (Choo 1989; Kuo 1989). The Center for Disease Control’s (CDC’s) third National Health and Nutrition Examination Study (NHANES III), conducted from 1988 to 1994, estimated that 1.8% of the United States population — or four million people — have been infected with HCV; 2.7 million remain chronically infected. Data from NHANES III may significantly underestimate the true prevalence of HCV infection in the United States since incarcerated and homeless individuals were not included in the populations surveyed. HCV prevalence among this country’s 1.8 million incarcerated persons is estimated at 30% to 40% (Reindollar 1999). A 2002 survey of 597 homeless veterans found an HCV seroprevalence of 41.7% (Cheung 2002). Epidemiological studies must include high-risk and high-prevalence populations to obtain accurate estimates of hepatitis C prevalence. The CDC’s Sentinel Counties Study of Viral Hepatitis provides data on the incidence of acute HCV infections. At present, only a pilot program — sentinel surveillance for physician-diagnosed chronic liver disease — tracks both acute and chronic HCV infections.
National surveillance of chronic hepatitis C infections is necessary to forecast disease burden and provide a sound basis for planning allocation of adequate resources for prevention, education, care, and treatment programs. The recommendations from CDC’s Guidelines for Viral Hepatitis Surveillance and Case Management should be implemented (CDC 2002).
1b. Increase access to sterile injection equipment.
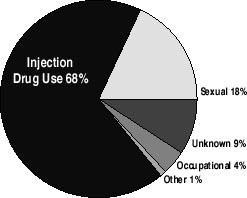
Although the majority of new HCV infections in the United States result from drug injection using shared, unsterilized equipment, it has not been widely and openly acknowledged that hepatitis C is a disease of drug users. HCV prevalence among injection drug users (IDUs) is estimated at 70% to 90% (Alter 1998; Donahue 1991; Garfein 1996; Thomas 1995). More than half of new hepatitis C infections are acquired through drug injection using shared, unsterilized equipment (Kim 2002). There is ample potential for HCV transmission among new IDUs via shared syringes and other injection drug equipment (Thorpe 2002; Vidal Trecan 2002).
Although CDC recommends that syringes and injection equipment never be shared or used more than once, its Guidelines for Prevention and Control of Hepatitis C Virus Infection (1998) and Guidelines for the Prevention of Opportunistic Infections Among HIV-Infected Persons (2002) state that shared injection equipment should be cleaned with bleach and water. The efficacy of bleach for neutralizing HCV has not been established (Hagen 2001).
Access to sterile syringes and injection equipment is vital to hepatitis C prevention. Research on the efficacy of bleach and identification of optimal disinfection practices for injection drug equipment is necessary as well. Policies that create barriers to risk reduction must be changed. Inadequate access to sterile syringes and injection equipment, restrictive one-for-one syringe exchange policies, limited access to methadone, and the unavailability of drug treatment on demand continue to fuel both the HCV and HIV epidemics. State and local barriers to syringe exchange programs must be removed; program expansion will require an increased commitment of resources and hence the overturning of the federal ban on syringe exchange funding. Legislation must be enacted to legalize pharmacy sale of syringes in the remaining states that prohibit over-the-counter sales without prescriptions, and all state and local public health programs must insure that pharmacy sale of syringes is accessible and affordable.
Sources of HCV Infection 1995-2000
1c. Provide HCV education for high-risk and high-prevalence populations.
More than three million people in the United States are injection drug users; 94,000 are between the ages of twelve and seventeen (National Household Survey on Drug Abuse, 2000 and 2001). The incidence of HCV infection is highest among new injectors, with an estimated 50% to 80% of injection drug users (IDUs) becoming infected within a year of initiating injection drug use (Garfein 1996). Education about HCV transmission must be provided to young people before they begin injecting drugs. Information about prevention, transmission, diagnosis, natural history, and treatment of HCV must be provided and integrated within program activities for staff and clients of detoxification facilities, drug treatment programs, shelters, methadone maintenance programs, correctional facilities, and AIDS service organizations. Hepatitis C advocacy organizations can provide HCV educational materials and information; AIDS service organizations can be an important source of HIV-related information for clients of hepatitis C organizations. Collaboration among these entities will benefit people with HCV, the coinfected, active and recovering drug users, the homeless, and others who are infected or at risk. Public health funding must be made available to support education, and state-contracted agencies must provide these services.
1d. Clarify the risk of non-injection drug use (e.g., snorting or smoking) associated HCV transmission.
Conflicting data have emerged about the risk of HCV infection from intranasal (i.e., snorting or sniffing) drug use (Conry Cantilena 1996; Murphy, 2000). There has also been speculation about HCV transmission from shared crack pipes, since frequent users often have burned or split lips from heated glass crack pipes. NHANES III participants were asked about drug-use history; however they were not specifically asked if they had ever injected drugs. Because drug-taking modes — snorting or smoking versus injecting — were not recorded in NHANES III, no estimate of the actual incidence of HCV transmission via intranasal drug use can be made from that data. A study of HCV-infected blood donors revealed a significant reluctance to disclose even one-time use of injection drugs. Forty-two percent (103) of 248 HCV-positive donors who disclosed intravenous drug use during a self-administered questionnaire about recreational drug use had denied any intravenous drug use during their initial blood donation screening (Conry Cantilena, 1996).
Research on the risk of intranasal drug use must clarify questions about this mode of drug administration as a potential route of transmission. Further investigation of the risk of HCV infection from smoking crack or other drugs is also needed. Studies must be designed to elicit accurate information about drug use. Pending more definitive data, educators and medical providers should incorporate appropriate and responsible messages on intranasal transmission risk.
1e. Clarify routes and risks of sexual HCV transmission.
HCV can be sexually transmitted, although the relative risk of sexual transmission, and by what means, remain controversial. Several studies have documented higher-than-average anti-HCV prevalence among men who have sex with men (MSM), sex workers, individuals who have had multiple partners, and partners of HIV/HCV coinfected individuals (Alter 1988; Alter 2002; Bodsworth 1996; Buchbinder 1994; Eyster 1991). Most research on sexual HCV transmission has not collected information about specific sexual acts.
Research on HCV transmission must employ direct questions about sexual behaviors. Mucosal transmission by oral, penile, vaginal, and anal routes must be investigated as well as sexual practices that may involve the exchange of blood. Information about the risk associated with specific sexual practices is needed to inform prevention program messages as well as individual decision-making about risk reduction to prevent HCV transmission.
1f. Sharpen the focus on mother-to-child HCV transmission.
Worldwide, 35% of those infected with hepatitis C are women in childbearing years. Infection through mother-to-infant transmission of HCV occurs in approximately 5% of children born to mothers with HCV (Yeung 2001). The risk of HCV transmission increases if the mother is coinfected with HIV or is a current or former injection drug user (Thomas 1998; Yeung 2001). At present, no interventions have been identified to prevent mother-to-infant transmission of HCV. Screening pregnant women for hepatitis C is not a routine part of prenatal care. The draft guidelines from the National Institute of Health’s Consensus Development Conference on Management of Hepatitis C: 2002 do not offer any guidance for HCV testing of infants or pregnant women. Voluntary testing and counseling for hepatitis C should be offered as part of routine prenatal care. Standardized diagnostic guidelines for mothers and infants are necessary both in clinical practice and in research settings. Further research to elucidate factors involved in mother-to-infant transmission of HCV is needed to identify risk-reduction and prevention strategies.
1g. Institute CDC’s recommendations for prevention of HCV transmission in hemodialysis facilities.
People receiving kidney dialysis are at risk for acquiring HCV infection when dialysis centers do not practice proper infection control procedures. A study that screened dialysis recipients for HCV antibodies at 40% of U.S. dialysis centers during December of 2000 reported that 8.4% tested positive (Tokars 2002). Other studies of dialysis recipients have reported anti-HCV prevalence ranging from 10% to 36% among adults and 18.5% among children (CDC 2001).
In April of 2001, the CDC issued recommendations for preventing transmission of pathogens among chronic hemodialysis patients; they included stricter infection control practices, regular monitoring of ALT levels, and HCV testing of dialysis recipients (CDC 2001). All dialysis facilities must implement these recommendations and be monitored by the appropriate licensing and regulatory bodies.
1h. Develop and implement HCV prevention strategies for the developing world.
Globally, an estimated 170 million people, or 3% of the world’s population, may be infected with hepatitis C (WHO 1999). HCV infections in the developing world are mainly acquired from unscreened, contaminated blood transfusions, unsterilized medical and dental equipment, and unsterilized instruments used for circumcision, scarification, tattooing, and traditional medicine. In parts of the developing world, injection drug use is also a major mode of HCV transmission. In some regions, it is estimated that 2.3 to 4.7 million new HCV infections occur each year as a result of unsafe injections (Kane 1999).
Prevention of new HCV infections must be a priority in resource-poor settings. This will include implementing screening of donor organs, blood, and blood products; offering training on viral inactivation techniques, infection control procedures, and proper methods of sterilizing medical equipment (including injection equipment); and promoting harm reduction and providing access to sterile injection equipment for injection drug users. Prevention interventions need to be adapted to specific regions, cultures, and settings.
2. Pathogenesis and Natural History
2a. Establish prospective, longitudinal cohort studies of the natural history of HIV/HCV coinfection in the era of HCV treatment and HAART.
It is estimated that 16-25% of HIV-positive individuals in the United States are coinfected with HCV, with substantially higher rates of coinfection among HIV-positive injection drug users (Thomas 2002; Sherman 2002). In the era of highly active antiretroviral therapy (HAART), HIV-related mortality has decreased significantly while HCV-related morbidity and mortality have become prominent in coinfected individuals. End-stage liver disease from HCV is now a leading cause of death in coinfected individuals (Bica 2001; Martín-Carbonero 2001; Monga 2001; Quintana 2002).
Pre-HAART-era data from cohorts of coinfected hemophiliacs and injection drug users indicate that coinfection with HIV produces an accelerated course of HCV disease (Eyster 1993; Rockstroh 1996; Sánchez Quijano 1995; Telfer 1994). In the HAART era, new questions and conflicting data about the efficacy of HAART in coinfected individuals have emerged (Greub 2000; Law 2002; Sulkowski 2002). We need to increase our understanding of the complex interrelationship between these two viruses, as well as the impact of HAART and immune restoration. So far, most studies that have examined the effect of HCV on responses to HAART and clinical progression of HIV disease have not collected information about the progression or severity of underlying HCV disease. Without data on actual liver histology, it is impossible to determine the severity of HCV disease, which may in turn affect an individual’s ability to respond to HAART.
Well-designed, prospective longitudinal cohorts will be essential to follow infected populations, define prognostic factors and other cofactors for progressive disease, observe changing treatment outcomes over time, and generate productive hypotheses for pathogenesis, prevention, and treatment studies. Because treatment modalities for HIV and HCV will continue to evolve, cohort studies must be large enough and long enough to measure and account for variations in treatment, as well as other cofactors such as access to health care, drug use, ethnicity, and gender. Barriers to enrollment such as invasive needle biopsies can be addressed by being restricted to intensified substudies, if necessary. In any case, full participation of coinfected persons and advocates will be essential in planning and implementing such cohort studies.
2b. Investigate the role of genetic and ethnic factors in susceptibility to HCV infection, disease progression, and response to treatment.
Hepatitis C infection is twice as prevalent among black Americans as white Americans. The highest observed prevalence of hepatitis C in the United States — a shocking 9.8% — occurs among black males aged 40 to 49 (Alter MJ 1999). African-Americans appear less likely to achieve spontaneous viral clearance of HCV (Thomas 2000; Villano 1999). In addition, race appears to have a substantial impact on the efficacy of interferon. Significantly lower treatment response rates have been observed in blacks than in whites, Latinos, or Asian-Americans (Jeffers 2002; McHutchison 2000; Reddy 1999). Research is needed to understand the mechanisms that account for these disparities and to identify strategies to improve treatment response.
2c. Investigate the role of sex differences in HCV disease progression.
High rates of spontaneous viral clearance have been observed in two cohorts of premenopausal women, and some evidence suggests that the course of hepatitis C disease in this population may be less severe (Benhamou 1999; Kenny-Walsh 1999; Poynard 1997; Weise 2000). No research has explored why female sex appears to be a favorable prognostic factor. The role of hormones, immunological differences between males and females, lower body mass, and socioeconomic factors all warrant further investigation.
2d. Investigate the influence of light-to-moderate alcohol consumption on HCV disease progression.
A large body of data has confirmed that alcohol consumption of more than 50 grams per day accelerates the progression of HCV-related liver disease (Harris 2002; Poynard 1997; Thomas 2000). The effect of light-to-moderate alcohol consumption on hepatitis C disease progression is unknown. Without more specific information, most clinicians simply recommend abstinence from alcohol; data to support or modify recommendations of abstinence are needed.
3. Diagnostics
3a. Educate primary care providers about hepatitis C infection, diagnosis, prevention, and treatment.
Acute hepatitis C infection is clinically silent for most infected people, with only 15% to 20% of individuals developing symptoms (Koretz 1993). Symptoms, when they occur — low-grade fever, fatigue, appetite loss, abdominal pain, nausea, and vomiting — are typical of many common viral infections. Chronic hepatitis C infection is also often asymptomatic, and both acute and chronic hepatitis C infections may go undiagnosed by physicians who fail to ask about the risk factors (Shehab 2001; Shehab 2002; Villano 1999).
Additionally, many physicians are unaware of the proper procedures for diagnosing hepatitis C (Shehab 1997). HIV-positive individuals (especially those with fewer than 200 CD4 cells), injection drug users, and transplant recipients may harbor occult hepatitis C infection (Beggren 2001; Beld 1999; Busch 2001; Lin 2002; Thomas 1995). Performing HCV RNA testing in these populations, even when an antibody test result is negative, can sometimes identify infections that might otherwise go undiagnosed. Professional educational programs, cross-disciplinary training, and public health initiatives are needed to increase knowledge of hepatitis C transmission, prevention, diagnostics, care, and treatment among health care providers at all levels.
3b. Continue research on non-invasive testing methods to replace or reduce the need for liver biopsy.
Liver biopsy is still the only way to assess the condition of liver tissue. Information from liver biopsy is used to assess the degree of inflammation, gauge hepatic cell death and damage, identify other causes of liver injury, and guide treatment decisions. Although fatalities from biopsy are extremely rare (0.01-0.1%), liver biopsy can be painful, and occasional complications such as hemorrhage or puncture of adjoining organs may occur. The risk of complications and the potential pain of the procedure have made liver biopsy unpopular with many patients. Sampling errors and variations among observers may result in serious diagnostic errors (Bejarno 2001). Providing sedation during the procedure can reduce associated pain, while using ultrasound to guide the biopsy reduces the risk of complications and sampling errors (Cadranel 2000; Pokorny 2002; Soyer 1993).
Alternatives to liver biopsy using panels of serum biochemical markers have been proposed and are under investigation. Although these tests may serve as substitutes when biopsy is contraindicated or refused, the information they yield is far less precise. The identification, development, and validation of a non-invasive, cost-effective replacement for liver biopsy would be an important breakthrough. In the meantime, to reduce the risk of pain, complications, and sampling errors, experienced physicians guided by ultrasound should perform liver biopsy, and pain management should be provided to those undergoing biopsy. A standardized and simplified system for evaluating the results of liver biopsy in research and clinical care settings should be instituted, and biopsies should be read by pathologists who are skilled at reviewing liver biopsies.
3c. Identify and validate prognostic markers and effective screening methods for early diagnosis of hepatocellular carcinoma (HCC).
Hepatocellular carcinoma (HCC) is a known complication of hepatitis C. In the United States, the incidence of HCC in the general population has increased from a rate of 1.4 cases per 100,000 in 1976-1980, to 2.4 cases per 100,000 during the period 1991-1995 (El-Serag 1999). This rise may reflect an epidemic of increased HCV transmission that occurred decades earlier. The annual incidence of HCC in hepatitis C-infected cirrhotics ranges from 1% to 4% (Di Bisceglie 1997; Lauer 2001).
HCC can be identified by measuring alpha-fetoprotein (AFP) levels and by ultrasound imaging, but the value of these tests for early detection of HCC in cirrhotic individuals has not been sufficiently demonstrated. The sensitivity and specificity of AFP levels in the detection of HCC varies considerably (from 39-64% and from 76-91%) in different studies (Collier 1997). Some research has shown that ultrasound surveillance increases early detection of HCC, but without reducing mortality (Larcos 1998; Solmi 1996). HCC mortality is extremely high, with five-year survival rates of less than 5% (El-Serag 1999). Better interventions to facilitate the early diagnosis of HCC and reduce the high fatality rate are urgently needed.
4. Care and Treatment
4a. Develop integrated, multidisciplinary systems of care for individuals with multiple co-morbidities (HCV, HIV, mental illness, addiction).
Individuals with hepatitis C are often grappling with other issues: HIV coinfection, the stress involved with illicit drug use, maintaining recovery from addiction, severe, debilitating fatigue, poverty, homelessness, or incarceration. HCV is more prevalent among the mentally ill; moreover, individuals with HCV have a greater prevalence of depression (Zdilar 2000).
Our health care system is not prepared to accommodate the needs of active users or dually and triply diagnosed individuals. Multidisciplinary systems of care have been proven successful in treating active users, coinfected individuals with addiction and psychiatric co-morbidities, and individuals in a methadone maintenance program (Backmund 2001; Schwartzapfel 2002; Sylvestre 2002). Cross-disciplinary care should become an integral part of the care and treatment of people living with HCV and HIV/HCV coinfection.
4b. Develop guidelines for the care and treatment of coinfected individuals.
No one has yet integrated the recommendations from the Guidelines for the Use of Antiretroviral Agents in HIV-Infected Adults and Adolescents (USPHS 2002) and the National Institutes of Health’s 2002 Consensus Statement on the Management of Hepatitis C (NIH 2002). Some infectious disease doctors providing care for both HIV and HCV may be more focused on HIV disease. Referral to a gastroenterologist is not always feasible and, if available, the gastro-enterologist may not be well informed about the clinical management of HIV. Adapting, integrating, and updating existing recommendations from the HIV-HCV International Panel under the aegis of an ongoing guidelines panel of the U.S. Public Health Service could address these concerns. Such guidelines would provide an essential resource for clinicians, treatment educators, treatment advocates, and coinfected individuals.
4c. Promote screening and vaccination for hepatitis A and hepatitis B among individuals infected with HCV or coinfected with HIV/HCV.
Individuals infected with HCV are at risk for severe, potentially fatal disease if they become coinfected with hepatitis A (Koff 2001; Pramoolsinsap 1999; Vento 1998; Vento 2000). Coinfection with hepatitis B may accelerate progression of an existing hepatitis C infection or even cause liver failure and death (Koff 2001; Liaw 2000). Because of these risks, CDC recommends vaccination against HAV and HBV for all individuals infected with or at risk for HCV infection. Yet many are not receiving vaccinations. A survey of primary care physicians found that only 1.6% of their HCV patients were vaccinated against HAV and only 3% had been vaccinated against HBV (Nicklin 1999).
Physicians, health educators, and direct service staff need to be educated about the importance of vaccination against HAV and HBV. Screening and vaccination initiatives are needed. Vaccination should be available in correctional facilities and outside of clinic and hospital-based settings, especially in venues such as syringe exchange programs, substance abuse treatment programs, shelters, and methadone maintenance clinics, where high-prevalence and high-risk groups receive services. Screening and vaccination should be provided free of charge. Public health funding must be available for these services.
4d. Develop strategies to enhance HAV and HBV vaccine immunogenicity in HIV-positive individuals.
Although vaccinations for hepatitis A and B are safe in HIV-positive individuals, vaccine immunogenicity is decreased, especially those with low CD4 cell counts (Bruguera 1992; Hess 1995; Neilson 1997; Puoti 2002). The CDC estimates that only 66-75% of HIV-positive individuals develop protective antibody responses after vaccination for HAV. The efficacy of HBV vaccination in HIV-positive individuals is unclear, and an optimal strategy to enhance immunogenicity is needed. Response testing after vaccination and use of additional doses have been proposed as possible interventions to improve HBV vaccine response in people with HIV (CDC 1993).
Research on interventions to enhance the immunogenicity of HAV and HBV vaccines in HIV-positive persons should be prioritized.
4e. Increase research of treatment safety and efficacy in understudied populations.
Most of the studies of HCV treatment efficacy and safety have focused on populations with favorable prognostic factors. Individuals with medical and psychiatric co-morbidities have been excluded from the pivotal studies of peg-interferon and ribavirin, and results from these trials may not be applicable to a majority of individuals with HCV infection. More research is urgently needed on the safety, efficacy, and optimal dosing and duration of HCV treatment in HIV-positive individuals, cirrhotics, African-Americans, active drug users, individuals on methadone maintenance, the mentally ill, transplant recipients, individuals with renal disease, individuals with autoimmune conditions, the elderly, young children, adolescents, and non-responders and relapsers after prior HCV treatment.
4f. Provide full access to hepatitis C care and treatment for all those in need.
Current treatments for HCV can cost up to $40,000 per year. The uninsured, underinsured, and those ineligible for patient assistance and entitlement programs go untreated, even when treatment is urgently needed. Cash-strapped AIDS Drug Assistance Programs (ADAPs) are unable to offer the latest HCV treatments; fewer than ten of them have the resources available to provide pegylated interferon and ribavirin.
Advocacy efforts to increase access to HCV treatment must continue. Entitlement programs and private insurers should cover the costs of HCV treatment, including laboratory monitoring and medications to manage its side effects. ADAPs must receive the necessary funding from Congress to cover HCV treatment. Strategies must be developed to provide coverage for HCV therapy among the uninsured who do not qualify for entitlements or patient assistance programs.
4g. Provide full access to hepatitis C prevention, care and treatment services for incarcerated individuals.
In the United States, 1.8 million individuals are incarcerated. HCV infection is endemic among prisoners; a 1994 study of HCV prevalence among 4,513 inmates (87% male; 13% female) in the California correctional system reported that 39.4% of the males and 53.5% of the females were HCV-antibody-positive (Ruiz 1999). The need for HCV treatment remains largely unmet in correctional systems. Policies about HCV treatment in prison differ in every state, and incarcerated individuals do not have uniform access to treatment for HCV. Some inmates have had to resort to legal action to obtain treatment. This is an unacceptable situation. State and national advocacy efforts to demand access to HCV treatment for inmates must be intensified.
CDC’s new guidance document on Prevention and Control of Infections With Hepatitis Viruses in Correctional Settings is a useful tool for advocates. CDC recommends medical evaluation of all inmates who are HCV antibody-positive and establishment of criteria for HCV treatment based on current treatment guidelines, and that treatment should be conducted in consultation with a specialist familiar with treatment regimens for HIV and HCV (CDC 2003).
4h. Increase research on strategies to manage side effects from HCV treatment.
The side effects of treatment for hepatitis C range from the uncomfortable to the life threatening. In a recent 1,100-person phase III trial of peg-interferon alfa-2a (with placebo or ribavirin) versus interferon alfa-2b, the rate of treatment discontinuation due to adverse events and/or laboratory abnormalities was 10% in the peg-interferon/ribavirin arm and 11% in the standard interferon arm. Dose reductions were necessary for 32% of the individuals in the peg-interferon/ribavirin arm (Fried 2002a). Significant dose reductions may have an impact on the response to treatment (Fried 2002a; McHutchison 2002).
A report on adverse events from a recent trial comparing peg-interferon alfa-2b and ribavirin to interferon alfa-2b and ribavirin found that more than 20% of participants in each arm experienced fatigue, headache, fever, muscle aches and stiffness, insomnia, nausea, hair loss, irritability, joint pain, loss of appetite and weight loss, depression, and injection site reactions (Manns 2001). The list of serious adverse events associated with interferon treatment, although occurring in fewer than 1% of individuals studied so far, is daunting and includes severe neuropsychiatric complications and suicidal ideation, as well as skin, kidney, blood, liver, heart, and autoimmune diseases and sensory organ disorders (Fried 2002b).
Research to increase the tolerability of, and adherence to, HCV treatment is a priority. More research is needed to identify the proper threshold to initiate the use of growth factors to treat anemia and neutropenia, and to study their impact on HCV treatment efficacy. Interventions to decrease neuro-pyschiatric side effects are a priority: the instruments used to screen for depression — both prior to initiation of HCV treatment and during treatment — have not been validated for this purpose. More exploration of the instruments used to diagnose depression and evaluation of the efficacy, side effects, and indications of selective serotonin reuptake inhibitors (SSRI), other antidepressants, and anti-anxiety agents is needed to optimize individual side effect management strategies.
4i. Identify optimal dosing strategies.
The two available pegylated interferons — peg-interferon alfa-2a (Pegasys, manufactured by Roche) and peg-interferon alfa-2b (Peg-Intron, manufactured by Schering-Plough) — use different types of PEG molecules to prolong the half-life of the interferon. Pegasys is premixed and given at a fixed dose, while Peg-Intron is dosed by weight and mixed prior to injection. Despite the lack of a head-to-head comparison, and despite the different methods of dosing, similar efficacy of the two products has been demonstrated. The overall rate of sustained virological response to treatment with Pegasys and Peg-Intron, combined with ribavirin, is 54% vs. 56%, respectively (Di Bisceglie 2002a).
Recent information from a pivotal study of Pegasys found that individuals with genotype 2 or genotype 3 responded to 24 weeks of therapy and a lower dose of ribavirin (800 mg) as well as those who received higher doses of ribavirin over 48 weeks. Individuals with genotype 1 obtained better responses with 48 weeks of treatment and a higher dose of ribavirin (1,000-1,200 mg) (Hadziyannis; unpublished data). With Peg-Intron, the recommended dose of ribavirin is 800 mg; this may be too low for individuals with genotype 1 and viral loads over 2,000,000 copies. The recommended duration of therapy (48 weeks) for Peg-Intron may be unnecessarily long for individuals with genotypes 2 and 3.
The full range and upper limit for weight-based dosing of Peg-Intron have not been adequately defined. Obese individuals typically have lower response rates, but it is unclear whether this is due to inadequate dosing of peg-interferon and ribavirin or to other poor prognostic factors, viral resistance, or a combination of these elements. For some, peg-interferon dosing may be too high, and dose reduction may be necessary to reduce hematologic problems.
In the absence of more effective and less toxic therapies for hepatitis C, questions about dosing variability must be addressed. More research needs to be conducted on optimal dosing and duration of therapy in other understudied populations, including individuals with acute hepatitis C infection, those with renal disease, advanced liver disease, non-responders to prior HCV treatment, those who have relapsed after treatment, children, individuals with autoimmune disorders, and transplant recipients.
5. Key Research Questions in HIV/HCV Coinfection
5a. Investigate sequencing of treatment for HIV and HCV.
It is still unclear when antiretroviral therapy should be initiated in coinfected individuals. Some studies have found a blunted immune response to HAART in coinfected individuals (Greub 2000; Law 2002). Earlier initiation of HAART may help to preserve immune function. End-stage liver disease (ESLD) occurs more frequently in individuals with low CD4 cell counts (Goedert 2002; Ragni 2001). Thus, for coinfected individuals, it is now critical to investigate whether earlier initiation of HAART — possibly earlier than today’s recommended thresholds of 200-350 CD4 cells — will decrease the incidence and progression of ESLD among coinfected individuals. Alternatively, since it may be possible to eradicate HCV in people who experience a sustained virological response (SVR) to therapy, early initiation of HCV treatment in HIV-positive individuals should also be explored. Preemptive treatment of HCV — even if an SVR is not achieved — may improve toleration of antiretroviral agents. Answering these questions will require allocation of resources for long-term treatment strategy studies.
5b. Establish a universal definition of hepatotoxicity and characterize its severity.
Coinfected individuals often have elevated liver enzyme levels, which may be due to liver disease, the hepatotoxicity of anti-HIV medications, or both (Staples 1999). A universal definition of hepatotoxicity for research and clinical practice is needed to increase the consistency and interpretability of results from clinical trials, to guide antiretroviral treatment decisions and to enable the collection of consistent adverse event data.
Hepatitis C coinfection significantly increases the risk of hepatotoxicity from HAART (Lana 2001; Nunez 2001). Some individuals must discontinue HAART altogether because of liver toxicity; in other instances, only one drug must be switched. Hepatotoxicity has multiple causes, such as underlying liver diseases unrelated to HCV, fatty liver, alcohol consumption, genetic variation, antiretrovirals, or other medications. We need more research to understand if HAART-related hepatotoxicity worsens HCV disease, and to differentiate between transient elevations in liver enzymes and clinically significant indications of clinical progression.
5c. Explore pharmacokinetics and drug levels in coinfected individuals.
Up to 90% of HIV-positive individuals receive at least one hepatotoxic drug (Orenstein 2002). The potential for drug interactions in HIV-positive individuals is abundant even without hepatitis C coinfection; often, these individuals may be taking lipid lowering agents, methadone, anti-anxiety medications, prophylaxis against opportunistic infections, vitamins, herbs, supplements, and antiretrovirals.
Several important antiretrovirals are metabolized by the liver. HCV-related liver damage may diminish the liver’s ability to metabolize these drugs. Drug levels may be elevated in individuals with liver disease, increasing side effects, interactions, and toxicities. The incidence of complications from antiretroviral therapy among coinfected individuals needs further investigation and documentation. The contribution of specific classes and drugs to interactions, side effects, and complications needs further study.
5d. Support access to and research on liver transplantation for HIV-positive individuals.
Although HAART has significantly increased the survival of HIV-positive individuals, their risk for end-stage liver disease remains significant. Some evidence has emerged in the HAART era indicating that HIV-positive individuals have post-transplantation outcomes equivalent to HIV-negative individuals (Gow 2001; Kuo 2001; Prachalias 2001; Ragni 1999). UNOS, the United Network for Organ Sharing, does not consider HIV infection to be a contra-indication for organ transplantation. Despite the emerging reports of favorable outcomes in HIV-positive individuals, insurers have sometimes denied reimbursement for transplants when HIV is involved, deeming it “experimental.” Expanding an indication to include people with HIV does not transform an established procedure into an “experiment.” Transplantation must be reimbursable for HIV-positive individuals.
Prospective studies of transplantation in HIV-positive individuals will provide vitally important information about the specific risks for those undergoing transplantation, as well as help to identify the optimal clinical management strategies for improved and extended survival of HIV-positive organ recipients.
6. Basic Research and Drug Development
6a. Support and intensify research into the molecular biology and immunopathogenesis of HCV.
The initial identification of hepatitis C (Choo 1989) ushered in a decade of productive virology and immunology research, as scientists began investigating the genetic structure of the virus, the role of viral proteins in HCV replication, the immune response to HCV, and how HCV causes disease. During the 1990s, researchers made significant inroads into understanding the structure and genetics of HCV. Scientists cloned the genome of various HCV strains (Choo 1991; Kato 1990; Takamizawa 1991) and characterized the morphology of HCV through isolation of virions using electron microscopy (Kaito 1994; Li 1995; Shimizu 1996). Researchers also discovered the crystal structure of the key viral proteins serine protease (Kim 1996; Love 1996), helicase (Cho 1998; Kim 1998; Yao 1997), and RNA-dependent RNA polymerase (Ago 1999). Immunologists identified major epitopes recognized by HCV-specific cytotoxic T lymphocytes (Battegay 1995; Cerny 1995; Kita 1993; Koziel 1993; Koziel 1995), cells which are believed to play a key role in liver injury and cell death. Other groups made preliminary attempts at developing a cell culture system to model the HCV replication cycle using infectious complementary DNA clones (Kholykhalov 1997; Yanagi 1997) and subgenomic RNA replicons (Lohmann 1999).
In recent years, researchers have provided detailed evaluations of cell-mediated immune responses to HCV (Day 2002; Godkin 2002; Lauer 2002; Penna 2002; Rosen 2002a) and further clarified the viral replication cycle and viral-host cell interactions (Bartenschlager 2002; Shirota 2002; Spahn 2001; Takikawa 2000; Tellinghuisen 2002; Walewski 2001). Researchers still lack an efficient, reproducible cell culture system that can support viral replication and infection. However, the refinement of cell culture models, including more robust replicon systems (Blight 2000; Ikeda 2002), as well as a surrogate tissue culture system using GBV-B in tamarin hepatocytes (Beames 2000), enables the examination of the expression and function of viral proteins. Replicon systems have facilitated drug development efforts by allowing researchers to screen compounds for activity against viral proteins involved in the replication cycle, with the significant limitation that these replicons cannot infect new cells. While chimpanzees are the only other animals aside from human beings known to support HCV replication, progress has been made towards making a viable chimeric mouse model of hepatitis C infection (Mercer 2001). Despite these advances, numerous challenges remain. Key aspects of HCV pathogenesis and the viral replication cycle are not fully understood, and further work on cell culture systems and animal models remains an urgent priority.
Continued elucidation of the pathogenesis of HCV will be critical in leading to new therapies. Researchers have not conclusively determined the receptors involved in HCV’s entry into cells, though the roles of CD81 (Meola 2000; Roccasecca 2003; Takikawa 2000), the low-density lipoprotein receptor (LDL-R) (Germi 2002; Hennig 2002; Wunschmann 2000), and most recently the asialoglycoprotein receptor (ASGP-R) (Saunier 2003), have been explored. The potential roles of HCV proteins — particularly the core protein and NS5A — in the pathogenesis of fibrosis progression, hepatic steatosis, and hepatocellular carcinoma require investigation (Gimenez-Barcons 2001; Shi 2002; Tsutsumi 2002; Yoshida 2001). Key foci in immunology research include clarifying the correlates of the protective immune responses that enable clearance of acute infection as well as defining mechanisms of long-term virological control in chronic infection (Bassett 2001; Major 2002; Shata 2002; Thomson 2003). Immunologists should also continue to explore the associations between HCV disease progression, cytokines such as IL-10 and TNF-alpha, and genetic polymorphisms (Rosen 2002b; Tokushige 2003; Yee 2001), and further investigate the possible contributions of interactions among HCV and gamma-delta T cells, NK cells, and dendritic cells to pathogenesis and immune evasion (Tseng 2001; Crotta 2002; Tseng 2002; Auffermann-Gretzinger 2001; Bain 2001).
Finally, efforts must be made to increase the utility of the mouse model and enhance the efficiency and reproducibility of in vitro cell culture systems. The chimeric mouse model incorporating human hepatocytes shows promise, though further work is necessary to better mimic the human immune response to HCV in this model. The National Institute of Allergy and Infectious Diseases (NIAID) Division of Microbiology and Infectious Diseases (DMID) “Request for Proposals for the Hepatitis Animal Model Network” (RFP-NIH-NIAID-DMID-99-19) provides a good vehicle for supporting small animal model studies and should be expanded to prioritize HCV research. DMID must also update its “Framework for Progress on Hepatitis C” (NIAID 1997) and increase its commitment to funding intramural and extramural basic research.
6b. Support and accelerate the development of new therapeutic strategies.
The standard HCV treatment using pegylated interferon and ribavirin has limited efficacy, suboptimal tolerability, and significant expense. New treatment options that are more potent, less toxic, and effective in those who relapse or do not respond to current regimens are desperately needed. Anecdotal reports suggest that many people with HCV are choosing — often based on the advice of their doctors — to defer treatment due to the limitations of interferon and ribavirin. The complicated calculus of when and whether to initiate HCV treatment begins with an assessment of one’s current disease state and risk of disease progression. However, for many, these considerations are superseded by the difficulties of managing treatment (including the duration of treatment, the necessity of injecting interferon, the risk of depression, and the potential impairment of quality of life) as well as the lower likelihood of a sustained virological response (SVR) among those with genotype 1, high viral loads, and HIV coinfection. New and better treatments could mitigate many of these concerns and make therapy for HCV more widely acceptable and, ultimately, more successful.
The mechanisms of action of interferon and ribavirin as treatment for HCV — as well as the causes of viral resistance and treatment failure — are not fully understood, and most likely involve multiple immunomodulatory and antiviral effects (Gretch 2001; Lau 2002; Taylor 2001). Researchers and drug companies are exploring several new viral targets and drug candidates informed by a substantial body of basic research into the molecular biology of HCV. Progress here has been delayed by the lack of standard tools to screen potential drugs — an efficient, reproducible cell culture system that can sustain viral replication and infection of new cells, and a small animal model. Chimpanzees, the only other animal model known to support HCV replication, have been used to study pathogenesis and immune response, yielding data that may inform efforts at finding a vaccine against HCV. However, chimpanzee research is prohibitively expensive and ethically challenging, and the supply of chimpanzees is extremely limited (Grakoui 2001; Lanford 2001; Lanford 2002).
Current drugs in development include those targeting translation initiation (antisense oligonucleotides and synthetic ribozymes), viral enzymes (serine protease and helicase), and inhibitors of RNA synthesis (RdRp inhibitors and IMPDH inhibitors), as well as antifibrotic compounds and immunomodulators (Di Bisceglie 2002b; Tan 2002). Most of these drugs are in very early stages of preclinical or clinical development. Vaccine development has not advanced beyond a few animal studies (Himoudi 2002; Matsui 2002; Pancholi 2003). During the 1990s, some drug and vaccine studies were delayed by lawsuits initiated by Chiron against other companies, claiming that their drug development programs infringed Chiron’s patents related to HCV (Cohen 1999). While these issues appear to be largely resolved for now, the potential inhibitory effect of intellectual property disputes on the development of new drugs and diagnostics calls for scrutiny and vigilance.
Speeding the development of new therapeutic strategies for HCV will require a coordinated effort involving government, industry, research institutions, private foundations supporting biomedical research, and HCV advocates, especially people infected with hepatitis C. A strategic partnership between public and private sectors could support exploration of new targets and a better understanding of viral-host interactions through techniques such as microarray analysis (Aizaki 2002; Bigger 2001), an intensive research and development program on tools for rapid and high-throughput screening of candidate compounds, preclinical research, and the establishment of a network to facilitate the recruitment of patients into clinical trials.
NIAID’s new Partnerships for Novel Approaches to Controlling Infectious Diseases, a collaboration between government, industry, and academia, has begun to focus on hepatitis B and should be expanded to address HCV. Increased leadership and coordination from the National Institutes of Health’s NIAID and National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), in concert with advocacy from HCV and HIV organizations, will be necessary to ensure progress on these fronts.
6c. Include HIV/HCV coinfected individuals in early-phase HCV treatment trials.
Preliminary data suggest a poorer response rate to HCV treatment in coinfected persons (Chung 2002; Perronne 2002). Because HCV is more aggressive in HIV-positive individuals, the need for new, more effective treatments is particularly urgent. Research on the safety and efficacy of HCV treatment in coinfected persons has lagged; usually, patients and clinicians must wait for a couple of years before these data are available to them. Coinfected individuals must be offered the opportunity to participate in clinical trials of new agents as soon as it is safe to do so. A good benchmark here would be to ensure enrollment of coinfected persons as soon as a safe and virologically active dose is defined.
6d. Establish a hepatitis C clinical research network.
The National Institutes of Health’s 2002 Consensus Statement on the Management of Hepatitis C calls for the establishment of a Hepatitis Clinical Research Network examining natural history, prevention, and treatment (NIH 2002). Such a network would fill a void in current research efforts, providing better data regarding pathogenesis, natural history, long-term clinical outcomes, and determinants of variations in response rates to current treatment. A clinical research network could also provide a mechanism to test new therapies alone and in combination with the standard of care. A well-designed network should support investigation into optimizing the use of current treatments in understudied populations, including injection drug users, cirrhotics, and people with a history of depression. A new network could systematically address many of the research recommendations in this document, specifically those in sections 1d and 1e (research on transmission routes); all of section 2 (Pathogenesis and Natural History); sections 3b and 3c (biopsy alternatives and HCC screening); sections 4e, 4h, and 4i (research on treatment in understudied populations, side effects, and dosing strategies); and all of section 5 (Key Research Questions in HIV/HCV Coinfection).
Much current knowledge on HCV and HIV/HCV coinfection has come from clinic-based cohort studies with relatively homogenous patient populations and treatment follow-up rates generally limited to five years at most. The existing multicenter networks that have studied HCV have a narrow focus or limited capacity for large-scale, long-term research. For instance, the Adult AIDS Clinical Trials Group (AACTG) and the American Foundation for AIDS Research (amfAR) have sponsored studies on HIV/HCV coinfection, while the industry-sponsored Hepatitis Research Network’s Clinical Trials Group has focused on treatment strategies. NIAID’s Division of Microbiology and Infectious Diseases (DMID) has begun to develop research networks, including the Hepatitis C Research Recovery Network and the Hepatitis C Cooperative Research Centers. This work should be supported and expanded, given the pressing need for increased resources, strong leadership, and strategic direction.
Appendix A: The 2000 Recommendations
Recommendations from TAG’s 2000 Hepatitis Report are listed below, with progress on achieving them and comments on their place in the current recommendations.
- CDC should further investigate the role of HCV sexual transmission in MSM.See recommendation 1e (Clarify routes and risks of sexual HCV transmission).
- CDC should update its 1998 recommendations to suggest HCV testing for all persons with HIV/AIDS.HCV testing has been recommended by CDC for all persons with HIV; also, see recommendation 3a (Educate primary care providers about HCV infection, diagnosis, prevention, and treatment).
- More research should be conducted to completely understand the immunologic responses associated with control of HCV infection.This is still an important recommendation; see recommendations 5a and 6a (Investigate sequencing of treatment for HIV and HCV and Support and intensify research into the molecular biology and immunopathogenesis of HCV).
- The NIAAA (National Institute on Alcohol Abuse and Alcoholism) should commence studies on the effects of alcohol in patients with HCV. The findings should be widely distributed to patients and community physicians in a timely manner.See recommendation 2d (Investigate the influence of light-to-moderate alcohol consumption on HCV disease progression).
- Large natural history studies should be initiated to determine the current natural history of HIV/HCV coinfected individuals in the era of HAART.This remains an important issue; see recommendation 2a (Establish prospective, longitudinal cohort studies of the natural history HIV/HCV coinfection in the era of HCV treatment and HAART).
- NIH’s ICDs (Institutes, Centers, and Divisions) [i.e., NIAID (National Institute of Allergy and Infectious Diseases), NIDDK (National Institute of Diabetes and Digestive and Kidney Diseases), NHLBI (National Heart, Lung, and Blood Institute)] should issue multiple RFAs for cross-training of fellows in hepatology and infectious disease/HIV research.Although this is a good idea, it may not be necessary — a hepatitis C clinical research network may be able to bring hepatologists and infectious disease researchers together. The AACTG has an integrated Liver Disease Subcommittee that allows for cross-disciplinary collaboration. See recommendation 6d (Establish a hepatitis C clinical research network).
- NIH’s Office of AIDS Research should make available some of its discretionary funding for basic and clinical research on HIV/HCV coinfection.OAR has included research on HCV coinfection in its strategic plan. Funding will be addressed in Version 2.0 of the full Hepatitis C/HIV Coinfection Report.
- The NIH should explore the desirability and feasibility of a Hepatitis Clinical Trials Network. The network would carry out phase I to phase IV clinical studies with nested basic science research.See recommendation 6d (Establish a hepatitis C clinical research network).
- Future HCV treatment trials should stratify for HIV serostatus and enroll both HIV-positive and HIV-negative people in order to gather these critical data.See recommendation 6c (Include HIV/HCV coinfected individuals in early-phase HCV treatment trials).
- HCV treatment should be mandated in all state and federal prison systems.Access to HCV treatment should be mandated; see recommendation 4g (Provide full access to hepatitis C care and treatment for incarcerated individuals).
- Transplant centers in the U.S. should consider HIV-positive people for liver transplantation.There have been successful liver transplants in HIV-positive recipients in the U.S; we are moving towards research to establish safety, efficacy, and the development of a standard of care for transplantation in HIV-positive individuals. See recommendation 5d (Support access to and research on liver transplantation for HIV-positive individuals).
- HCV patients must have access to their HCV RNA levels at timely intervals (e.g., week 24) while on HCV treatment studies.This is an important concern; new data about 12-week early stopping rules in individuals with HCV monoinfection, and the longer half-life of HCV virions in HIV/HCV coinfected individuals should be considered in study design and reimbursement.
- Schering-Plough must unbundle Rebetron® so that ribavirin can be purchased separately.Unfortunately, this has not made HCV treatment more financially accessible unless individuals are purchasing their ribavirin from a compounding pharmacy. See recommendation 4f (Provide full access to hepatitis C care and treatment for all those in need).
- Research should be conducted to determine the lowest effective dose of ribavirin to minimize unnecessary toxicity.Although issues about ribavirin dosing have been fairly well resolved, issues about the dosing of peg-interferon remain. See recommendations 4h and 4i (Increase research on strategies to manage side effects from HCV treatment; and Identify optimal dosing strategies).
- All 50 U.S. states should add ribavirin to their Medicaid and ADAP formularies.Currently, Medicaid programs in all 50 states cover ribavirin and Schering’s Peg-Intron®; however, serious access problems remain. See recommendation 4f (Provide full access to hepatitis C care and treatment for all those in need).
- Industry should conduct drug interaction studies of anti-HIV drugs in HIV/HCV coinfected people while drugs are in development so that potential hepatotoxicity and drug interactions are defined prior to approval.See recommendations 5b, 5c, and 6c (Establish a universal definition of hepatoxicity and characterize its severity; Explore pharmacokinetics and drug levels in coinfected individuals; and Include HIV/HCV coinfected individuals in early-phase HCV treatment trials).
- FDA should grant Hoffmann-La Roche’s PEG-IFN NDA (new drug application) a “priority review” because of the unmet need for therapies for HCV patients with cirrhosis.Roche’s Pegasys® was approved in October of 2002.
- HCV-treating physicians should fully explain the risk and benefits of IFN/RBV combination therapy with their patients as well as estimates of treatment response according to host and viral characteristics.See recommendation 3a (Educate primary care providers about HCV infection, prevention, and treatment).
- Industry must actively recruit African-Americans in all phases of HCV clinical trials. These studies should have the statistical power to assess racial differences in viral clearance and response rates.NIH is currently funding a study on HCV treatment responses in African-Americans; Roche has an ongoing safety and efficacy study as well. See recommendation 2b (Investigate the role of genetic and ethnic factors in susceptibility to HCV infection, progression, and response to treatment).
- Hepatitis treatment advocates should be included in all facets of NIH decision-making about hepatitis clinical and basic science research, including protocol development, scientific agenda committees, and grant reviews.See recommendations 6a and 6d (Support and intensify research into the molecular biology and immunopathogenesis of HCV infection; and Establish a hepatitis C clinical research network).
References
- Ago H, Adachi T, Yoshida A, et al. Crystal structure of the RNA-dependent RNA polymerase of hepatitis C virus. Structure Fold Des 7(11):1417-26. 1999.
- Aizaki H, Harada T, Otsuka M, et al. Expression profiling of liver cell lines expressing entire or parts of hepatitis C virus open reading frame. Hepatology 36(6):1431-8. 2002.
- Alter MJ, Kruszon-Moran D, Nainan OV, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med 341:556-62. 1999.
- Alter MJ, Moyer LM. The importance of preventing hepatitis C virus infection among injection drug users in the United States. J Acquir Immune Defic Syndr 18 (S1): S6-S10. 1998.
- Alter MJ. Prevention of spread of hepatitis C. Hepatology 36 (5, Suppl 1): S93-S98. 2002.
- Auffermann-Gretzinger S, Keeffe EB, Levy S. Impaired dendritic cell maturation in patients with chronic, but not resolved, hepatitis C virus infection. Blood 15;97(10):3171-6. 2001.
- Backmund M, Meyer K, Von Zielonka M, et al. Treatment of hepatitis C infection in injection drug users. Hepatology 34(1):188-193. 2001.
- Bain C, Fatmi A, Zoulim F, et al. Impaired allostimulatory function of dendritic cells in chronic hepatitis C infection. Gastroenterology 120(2):512-24. 2001.
- Bartenschlager R, Lohmann V. Replication of hepatitis C virus. J Gen Virol 81(Pt 7):1631-48. 2000.
- Bassett SE, Guerra B, Brasky K, et al. Protective immune response to hepatitis C virus in chimpanzees rechallenged following clearance of primary infection. Hepatology 33(6):1479-87. 2001.
- Battegay M, Fikes J, Di Bisceglie AM, et al. Patients with chronic hepatitis C have circulating cytotoxic T cells which recognize hepatitis C virus-encoded peptides binding to HLA-A2.1 molecules. J Virol 69(4):2462-70. 1995.
- Beames B, Chavez D, Guerra B, et al. Development of a primary tamarin hepatocyte culture system for GB virus-B: a surrogate model for hepatitis C virus. J Virol 74(24):11764-72. 2000.
- Bejarno PA, Koehler A, Sherman KE. Second opinion in liver biopsy. Am J Gastroenterol 96 (11): 3158-64. 2001.
- Beld M, Penning M, van Putten M, et al. Low levels of hepatitis C virus RNA in serum, plasma and peripheral blood mononuclear cells of injecting drug users during long antibody-undetectable periods before seroconversion. Blood 94 (4): 1183-91. 1999.
- Benhamou Y, Bochet M, Di Martino V, et al. Liver fibrosis progression in human immunodeficiency virus and hepatitis C coinfected patients. Hepatology 30(4):1054-58. 1999.
- Berggren R, Jain M, Hester J, et al. (abstract #562) False-negative hepatitis C antibody is associated with low CD4+ cell counts in HIV/HCV-coinfected individuals. 8th Conference on Retroviruses and Opportunistic Infections, Chicago Illinois, 2001.
- Bica I, McGovern B, Dhar R, et al. Increasing mortality due to end-stage liver disease in patients with human immunodeficiency virus infection. Clin Infect Dis 32 (3): 492-97. 2001.
- Bigger CB, Brasky KM, Lanford RE. DNA microarray analysis of chimpanzee liver during acute resolving hepatitis C virus infection. J Virol 75(15):7059-66. 2001.
- Blight KJ, Kolykhalov AA, Rice CM. Efficient initiation of HCV RNA replication in cell culture. Science 290(5498):1972-4. 2000.
- Bodsworth NJ, Cunningham P, Kaldor J, et al. Hepatitis C virus infection in a large cohort of homosexually active men: independent associations with HIV-1 infection and injecting drug use but not sexual behavior. Genitourin Med 72(118):118-122. 1996.
- Bruguera M, Cremades M, Salinas R et al. Impaired response to recombinant hepatitis B vaccine in HIV-infected persons. J Clin Gastroenterol 14(1):27-30. 1992.
- Buchbinder SP, Katz MH, Hessol NA, et al. Hepatitis C virus in sexually active homosexual men. J Infection 29:263-269. 1994.
- Busch MP, Laycock ME, Mohr, et al. (abstract #235) Failure of serologic assays for diagnosis of hepatitis B and C virus infections in patients with advanced HIV. 8th Conference on Retroviruses and Opportunistic Infections. Chicago, Illinois, 2001.
- Cadranel JF, Rufat P, Degos F. Practices of liver biopsy in France: results of a prospective nationwide survey. For the Group of Epidemiology of the French Association for the Study of the Liver (AFEF). Hepatology 32(3): 477-81. 2000.
- Centers for Disease Control and Prevention. Recommendations of the Advisory Committee on Immunization Practices (ACIP): Use of vaccines and immune globulins in persons with altered immunocompetence. MMWR Recommendations and Reports 42 (No. RR-4). www.cdc.gov/mmwr/preview/mmwrhtml/00023141.htm. 1993.
- Centers for Disease Control and Prevention. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR:47 (No. RR-19). www.cdc.gov/mmwr/preview/mmwrhtml/00055154.htm. 1998.
- Centers for Disease Control and Prevention. Alter MJ, Lyerla RL, Tokars JI, et al. Recommendations for preventing transmission among chronic hemodialysis patients. MMWR Recommendations and Reports 50 (RR05);1-43. www.cdc.gov/mmwr/preview/mmwrhtml/rr5005a1.htm. April 27, 2001.
- Centers for Disease Control and Prevention. Guidelines for Viral Hepatitis Surveillance and Case Management. Atlanta, GA June, 2002.
- Centers for Disease Control and Prevention. Prevention and Control of Infections with Hepatitis Viruses in Correctional Settings. MMWR Recommendations and Reports 52 (RR01);1-33. www.cdc.gov/mmwr/preview/mmwrhtml/rr5201a1.htm. January 24, 2003.
- Cerny A, McHutchison JG, Pasquinelli C, et al. Cytotoxic T lymphocyte response to hepatitis C virus-derived peptides containing the HLA A2.1 binding motif. J Clin Invest 95(2):521-30. 1995.
- Cheung RC, Hanson AK, Maganti K, et al.Viral hepatitis and other diseases in a homeless population. J Clin Gastroenterol 34 (4): 476-80. 2002.
- Cho HS, Ha NC, Kang LW, et al. Crystal structure of RNA helicase from genotype 1b hepatitis C virus. A feasible mechanism of unwinding duplex RNA. J Biol Chem 273(24):15045-52. 1998.
- Choo QL, Kuo G, Weiner AJ, et al. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science 244:359-362. 1989.
- Choo QL, Richman KH, Han JH, et al. Genetic organization and diversity of the hepatitis C virus. Proc Natl Acad Sci USA 88:2451-2555. 1991.
- Chung RT, Anderson J, Alston B, et al. (abstract LB15) A randomized, controlled trial of pegylated interferon alpha-2a with ribavirin vs. interferon alpha-2a with ribavirin for the treatment of chronic HCV in HIV co-infection: ACTG 5071. 9th Conference on Retroviruses and Opportunistic Infections. Seattle, Washington, 2002b.
- Cohen J. The scientific challenge of hepatitis C. Science 285(5424):26-30. 1999.
- Collier J, Sherman M. Screening for hepatocellular carcinoma. Hepatology 27 (1): 273-78. 1998.
- Conry-Cantilena C, VanRaden M, Gibble J, et al. Routes of infection, viremia and liver disease in blood donors found to have hepatitis C virus infection. N Engl J Med 334: 1691-1696. 1996.
- Crotta S, Stilla A, Wack A, D’Andrea A, et al. Inhibition of natural killer cells through engagement of CD81 by the major hepatitis C virus envelope protein. J Exp Med 195(1):35-41. 2002.
- Day CL, Lauer GM, Robbins GK, et al. Broad specificity of virus-specific CD4(+) T-helper-cell responses in resolved hepatitis C virus infection. J Virol 76(24):12584-95. 2002.
- Di Bisceglie AM. Hepatitis C and hepatocellular carcinoma. Hepatology 26(3 Suppl 1): 34S-38S. 1997.
- Di Bisceglie AM, Hoofnagle JH. Optimal therapy of hepatitis C. Hepatology 36 (5 Suppl 1): S 121-S127. 2002a.
- Di Bisceglie AM, McHutchison J, Rice CM. New therapeutic strategies for hepatitis C. Hepatology 35(1):224-31. 2002b.
- Donahue JG, Nelson KE, MuZoz A, et al. Antibody to hepatitis C virus among cardiac surgery patients, homosexual men and intravenous drug users in Baltimore, Maryland. Am J Epidemiology 134(10): 1206-1211. 1991.
- El-Serag HB, Mason AC, et al. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med 340(10):745-50. 1999.
- Eyster ME, Alter HJ, Aledort LM, et al. Heterosexual transmission of hepatitis C virus (HCV) and human immunodeficiency virus (HIV). Ann Intern Med 115(10):764-8. 1991.
- Eyster ME, Diamondstone LS, Lien J-M, et al. Natural history of hepatitis C virus infection in multitransfused hemophiliacs: effect of coinfection with human immunodeficiency virus. J Acquir Immune Defic Syndr 6(6):602-610. 1993.
- Fried MW, Shiffman ML, Reddy RK, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 347(13):975-82. 2002a.
- Fried MW. Side effects of therapy of hepatitis C and their management. Hepatology 36(5 Suppl 1):S237-244. 2002b.
- Garfein RS, Vlahov D, Galai N, et al. Viral infections in short-term injection drug users: the prevalence of the hepatitis C, hepatitis B, human immunodeficiency, and human T-lymphotropic viruses. Am J of Public Health 86(5):655-661. 1996.
- Germi R, Crance JM, Garin D, et al. Cellular glycosaminoglycans and low density lipoprotein receptor are involved in hepatitis C virus adsorption. J Med Virol 68(2):206-15. 2002.
- Gimenez-Barcons M, Franco S, Suarez Y, et al. High amino acid variability within the NS5A of hepatitis C virus (HCV) is associated with hepatocellular carcinoma in patients with HCV-1b-related cirrhosis. Hepatology 34(1):158-67. 2001.
- Godkin AJ, Thomas HC, Openshaw PJ. Evolution of epitope-specific memory CD4(+) T cells after clearance of hepatitis C virus. J Immunol 169(4):2210-4. 2002.
- Goedert JJ, Eyster ME, Lederman MM, et al. End-stage liver disease in persons with hemophilia and transfusion-associated infections. Blood 100(5):1584-89. 2002.
- Gow PJ, Multimer D. (Correspondence) Liver transplantation for an HIV-positive patient in the era of highly active antiretroviral therapy. AIDS 15(2): 291-2. 2001.
- Grakoui A, Hanson HL, Rice CM. Bad time for Bonzo? Experimental models of hepatitis C virus infection, replication, and pathogenesis. Hepatology 33(3):489-95. 2001.
- Gretch D. Mechanism of interferon resistance in hepatitis C. Lancet 358(9294):1662-4. 2001.
- Greub G, Ledergerber M, Battegay P, et al. Clinical Progression, survival and immune recovery during antiretroviral therapy in patients with HIV-1 and hepatitis C virus coinfection: the Swiss HIV cohort study. Lancet 356:1800-1805. 2000.
- Hagan H, Thiede H, Weiss N, et al. Sharing of drug preparation equipment as a risk factor for hepatitis C. Am J Public Health 91(1):42-46. 2001.
- Harris H, Ramsay ME, Andrews N, et al. Clinical course of hepatitis C virus during the first decade of infection: cohort study. BMJ 324: 1-6. 2002.
- Hennig BJ, Hellier S, Frodsham AJ, et al. Association of low-density lipoprotein receptor polymorphisms and outcome of hepatitis C infection. Genes Immun 3(6):359-67. 2002
- Hess G, Clemens R, Bienzle U et al. Immunogenicity and safety of an inactivated hepatitis A vaccine in anti-HIV positive and negative homosexual men. J Med Virol 46(1):40-2. 1995.
- Himoudi N, Abraham JD, Fournillier A, et al. Comparative vaccine studies in HLA-A2.1-transgenic mice reveal a clustered organization of epitopes presented in hepatitis C virus natural infection. J Virol 76(24):12735-46. 2002.
- Ikeda M, Yi M, Li K, Lemon SM. Selectable subgenomic and genome-length dicistronic RNAs derived from an infectious molecular clone of the HCV-N strain of hepatitis C virus replicate efficiently in cultured Huh7 cells. J Virol 76(6):2997-3006. 2002.
- Jeffers LJ, Cassidy W, Howell C, et al. (abstract ID 102794) Peginterferon alfa-2a (40KD0 (Pegasys¨) in combination with ribavirin in African American and Caucasian patients with HCV genotype 1: an interim report of a comparitive, multicenter, efficacy and safety study. American Association for the Study of Liver Diseases. Boston, Massachusetts 2002.
- Kaito M, Watanabe S, Tsukiyama-Kohara K, et al. Hepatitis C virus particle detected by immunoelectron microscopic study. J Gen Virol 75(Pt 7):1755-60. 1994.
- Kane A, Lloyd J, Zaffran M. Transmission of hepatitis B, hepatitis C and human immunodeficiency viruses through unsafe injections in the developing world: model-based regional estimates. Bull World Health Organ 77(10):801-07. 1999.
- Kato N, Hijikata M, Ootsuyama Y, et al. Molecular cloning of the human hepatitis C virus genome from Japanese patients with non-A, non-B hepatitis. Proc Natl Acad Sci USA 87(24):9524-8. 1990.
- Kenny-Walsh E. Clinical outcomes after hepatitis C infection from contaminated anti-D immune globulin. N Engl J Med 340(16): 228-33. 1999.
- Kim JL, Morgenstern KA, Griffith JP, et al. Hepatitis C virus NS3 RNA helicase domain with a bound oligonucleotide: the crystal structure provides insights into the mode of unwinding. Structure 6(1):89-100. 1998.
- Kim JL, Morgenstern KA, Lin C, et al. Crystal structure of the hepatitis C virus NS3 protease domain complexed with a synthetic NS4A cofactor peptide. Cell 87(2):343-55. 1996.
- Kim WR, Brown RS Jr, Terrault, NA, et al. Burden of liver disease in the United States: summary of a workshop. Hepatology 36 (1): 227-42. 2002a.
- Kinzie JL, Naylor PH, Nathani MG, et al. African Americans with genotype 1 treated with interferon for chronic hepatitis C have a lower end of treatment response than Caucasians. J Viral Hepat 8(4):264-69. 2001.
- Kita H, Moriyama T, Kaneko T, et al. HLA B44-restricted cytotoxic T lymphocytes recognizing an epitope on hepatitis C virus nucleocapsid protein. Hepatology 18(5):1039-44. 1993.
- Koff RS. Risks associated with hepatitis A and hepatitis B in patients with hepatitis C. J Clin Gastroenterol 33(1):20-6. 2001.
- Kolykhalov AA, Agapov EV, Blight KJ, et al. Transmission of hepatitis C by intrahepatic inoculation with transcribed RNA. Science 277(5325):570-4. 1997.
- Koretz RL, Abbey H, Coleman E, et al. Non-A, non-B post-transfusion hepatitis. Ann Intern Med 119:110-115. 1993.
- Koziel MJ, Dudley D, Afdhal N, et al. Hepatitis C virus (HCV)-specific cytotoxic T lymphocytes recognize epitopes in the core and envelope proteins of HCV. J Virol 67(12):7522-32. 1993.
- Koziel MJ, Dudley D, Afdhal N, et al. HLA class I-restricted cytotoxic T lymphocytes specific for hepatitis C virus. Identification of multiple epitopes and characterization of patterns of cytokine release. J Clin Invest 96(5):2311-21. 1995.
- Kuo G, Choo Q-L, Alter HJ, et al. An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis. Science: 244:362-364. 1989.
- Kuo PC, Stock PG. Transplantation in the HIV+ patient. Am J Transplant 1(1):13-7. 2001.
- Lana R, Nunez M, Mendoza JL, et al. Rate and risk factors of liver toxicity in patients receiving antiretroviral therapy. Med Clin (Barc) 117(16):607-10. 2001.
- Lanford RE, Bigger C, Bassett S, Klimpel G. The chimpanzee model of hepatitis C virus infections. ILAR J 42(2):117-26. 2001.
- Lanford RE, Bigger C. Advances in model systems for hepatitis C virus research. Virology 293(1):1-9. 2002.
- Larcos G, Sorokopud H, Berry G, et al. Sonographic screening for hepatocellular carcinoma in patients with chronic hepatitis or cirrhosis: an evaluation. Am J Roentgenology 171:433-35. 1998.
- Lau JY, Tam RC, Liang TJ, Hong Z. Mechanism of action of ribavirin in the combination treatment of chronic HCV infection. Hepatology 35(5):1002-9. 2002.
- Lauer GM, Ouchi K, Chung RT, et al. Comprehensive analysis of CD8(+)-T-cell responses against hepatitis C virus reveals multiple unpredicted specificities. J Virol 76(12):6104-13. 2002.
- Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med 345(1):41-52. 2001.
- Law P, Duncombe C, Dore G, et al. (abstract 661-M) Hepatitis viruses: Influence on morbidity, mortality and response to antiretroviral therapy. 9th Conference on Retroviruses and Opportunistic Infections. Seattle, Washington, 2002.
- Li X, Jeffers LJ, Shao L, et al. Identification of hepatitis C virus by immunoelectron microscopy. J Viral Hepat 2(5):227-34. 1995.
- Liaw YF, Yeh CT, Tsai SL. Impact of acute hepatitis B superinfection on chronic hepatitis C virus infection. Am J Gastroenterol 95(10):2978-80. 2000.
- Lin HH, Chiou HM, Lee SSJ et al. (abstract WePeB5978) Use of Cobas Amplicator HCV test for the detection of HCV RNA in HIV-1 infected patients. XIV International AIDS Conference. Barcelona, Spain. 2002.
- Lohmann V, Korner F, Koch J, et al. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science 285(5424):110-3. 1999.
- Love RA, Parge HE, Wickersham JA, et al. The crystal structure of hepatitis C virus NS3 proteinase reveals a trypsin-like fold and a structural zinc binding site. Cell 87(2):331-42. 1996.
- Major ME, Mihalik K, Puig M, et al. Previously infected and recovered chimpanzees exhibit rapid responses that control hepatitis C virus replication upon rechallenge. J Virol 76(13):6586-95. 2002.
- Manns MP, McHutchison JG, Gordon SC et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for the initial treatment of chronic hepatitis C: a randomized trial. Lancet 358 (9286): 958-65. 2001
- Martín-Carbonero L, Soriano V, Valencia E, et al. Increasing impact of chronic viral hepatitis on hospital admissions and mortality among HIV-infected patients. AIDS Res Human Retrov 17 (16): 1467-71. 2001.
- Matsui M, Moriya O, Abdel-Aziz N, et al. Induction of hepatitis C virus-specific cytotoxic T lymphocytes in mice by immunization with dendritic cells transduced with replication-defective recombinant adenovirus. Vaccine 21(3-4):211-20. 2002.
- McHutchison JG, Manna M, Patel K, et al. Adherence to combination therapy enhances sustained responses in genotype-1 infected patients with chronic hepatitis C. Gastroenterology 123 (4): 1061-9. 2002.
- McHutchison JG, Poynard T, Pianko S, et al. The impact of interferon plus ribavirin on response to therapy in black patients with chronic hepatitis C. Gastroenterology 119(5):1317-23. 2000.
- Meola A, Sbardellati A, Bruni Ercole B, et al. Binding of hepatitis C virus E2 glycoprotein to CD81 does not correlate with species permissiveness to infection. J Virol 74(13):5933-8. 2000.
- Mercer DF, Schiller DE, Elliott JF, et al. Hepatitis C virus replication in mice with chimeric human livers. Nat Med 7(8):927-33. 2001.
- Monga HK, Rodriguez-Barradas MC, Breaux K, et al. Hepatitis C virus infection-related morbidity and mortality among patients with human immunodeficiency virus infection. Clin Infect Dis 33:240-47. 2001.
- Murphy EL, Bryzman SM, Glynn SA, et al. Risk factors for hepatitis C virus infection in United States blood donors. Hepatology 31(3):756-762. 2000.
- National Institute of Allergy and Infectious Diseases (NIAID), Division of Microbiology and Infectious Diseases (DMID). NIAID’s Framework for Progress on Hepatitis C. www.niaid.nih.gov/dmid/meetings/framework.htm — last updated 2001.
- National Institutes of Health Consensus Development Conference Statement. Management of Hepatitis C: 2002. http://consensus.nih.gov/cons/116/116cdc_intro.htm. Final statement September 12, 2002.
- Neilsen GA, Bodsworth NJ, Watts N. Response to hepatitis A vaccination in human immunodeficiency virus-infected and -uninfected homosexual men. J Infect Dis 176(4):1064-67. 1997.
- Nicklin DE, Schultz C, Brensinger CM, et al. Current care of hepatitis C-positive patients by primary care physicians in an integrated delivery system. J Am Board Fam Pract 12(6):427-35. 1999.
- Nunez M, Lana R, Mendoza JL, et al. Risk factors for severe hepatic injury after introduction of highly active antiretroviral therapy. J Acquir Immune Defic Syndr 27(5):426-31. 2001.
- Orenstein R, Tsogas N. Looking beyond highly active antiretroviral therapy: drug-related hepatotoxicity in patients with human immunodeficiency virus infection. Pharmacotherapy 22 (11):1468-78. 2002.
- Pancholi P, Perkus M, Tricoche N, et al. DNA immunization with hepatitis C virus (HCV) polycistronic genes or immunization by HCV DNA priming-recombinant canarypox virus boosting induces immune responses and protection from recombinant HCV-vaccinia virus infection in HLA-A2.1-transgenic mice. J Virol 77(1):382-90. 2003.
- Penna A, Missale G, Lamonaca V, et al. Intrahepatic and circulating HLA class II-restricted, hepatitis C virus-specific T cells: functional characterization in patients with chronic hepatitis C. Hepatology 35(5):1225-36. 2002.
- Perronne C, Carrat F, Bani Sadr T, et al. (abstract LBOr16) RIBAVIC trial (ANRS HC02): a controlled randomized trial of pegylated interferon alfa-2b plus ribavirin vs. interferon alfa-2b plus ribavirin for the initial treatment of chronic hepatitis C in HIV co-infected patients: preliminary results. XVI International AIDS Conference. Barcelona, Spain 2002.
- Pokorny CS, Waterland M. Short-stay, out-of-hospital radiologically guided liver biopsy. Med J Australia 176:67-69. 2002.
- Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR and DOSVIRC groups. Lancet 349: (9055):825-32. 1997.
- Prachalias AA, Pozniak A, Taylor C, et al. Liver transplantation in adults coinfected with HIV. Transplantation 72(10):1684-88. 2001.
- Pramoolsinsap C, Poovorawan Y, Hirsch P, et al. Acute, hepatitis-A super-infection in HBV carriers, or chronic liver disease related to HBV or HCV. Ann Trop Med Parasitol 93(7):745-51. 1999.
- Puoti M, Airoldi M, Bruno R. Hepatitis B virus co-infection in human immunodeficiency virus-infected subjects. AIDS Rev 2002 4(1):27-35. 2002.
- Quintana M, Barrasa A, Del Amo J, et al. (abstract ThPeC7511) Morbidity and mortality by hepatitis C virus (HCV) infection and HCV genotype in a cohort of HIV positive hemophiliacs in Madrid: 1979 to 2000. XIV International AIDS Conference. Barcelona, Spain 2002.
- Ragni MV, Belle SH. Impact of human immunodeficiency virus infection on progression to end-stage liver disease in individuals with hemophilia and hepatitis C virus infection. J Infect Dis 183: 1112-15. 2001.
- Ragni MV, Dodson SF, Hunt SC, et al. (Correspondence) Liver transplantation in a hemophilia patient with acquired immune deficiency syndrome. Blood 93:1113-14. 1999.
- Reddy RK, Hoofnagle JH, Tong MJ, et al. Racial differences in responses to therapy with interferon in chronic hepatitis C. Hepatology 30(3):787-93. 1999.
- Reindollar RW. Hepatitis C and the correctional population. Am J Med 107 (6B): 100S-103S. 1999.
- Roccasecca R, Ansuini H, Vitelli A, et al. Binding of the Hepatitis C Virus E2 Glycoprotein to CD81 Is Strain Specific and Is Modulated by a Complex Interplay between Hypervariable Regions 1 and 2. J Virol 77(3):1856-67. 2003.
- Rockstroh JK, Spengler U, Sudhop T, et al. Immunosuppression may lead to progression of hepatitis C virus-associated liver disease in hemophiliacs coinfected with HIV. Am J Gastroenterol 91(12):2563-68. 1996.
- Rosen HR, McHutchison JG, Conrad AJ, et al. Tumor necrosis factor genetic polymorphisms and response to antiviral therapy in patients with chronic hepatitis C. Am J Gastroenterol 97(3):714-20. 2002.
- Rosen HR, Miner C, Sasaki AW, et al. Frequencies of HCV-specific effector CD4+ T cells by flow cytometry: correlation with clinical disease stages. Hepatology 35:190-198. 2002.
- Ruiz JD, Molitor F, Sun RK, et al. Prevalence and correlates of Hepatitis C Infection among inmates entering the California Correctional system. Western J of Medicine; 170(3):156-60. 1999.
- Sánchez-Quijano A, Andreu J, Gavilán, et al. Influence of human immunodeficiency virus type 1 infection on the natural course of chronic parenterally acquired hepatitis C. Eur J Clin Microbiol Infect Dis 14(11):949-53. 1995.
- Saunier B, Triyatni M, Ulianich L, et al. Role of the asialoglycoprotein receptor in binding and entry of hepatitis C virus structural proteins in cultured human hepatocytes. J Virol 77(1):546-59. 2003.
- Schwartzapfel B, Taylor LE, MacLeod Er, et al. (abstract ThPeC7512) A pilot program for treating hepatitis C in HIV/hepatitis C virus coinfected individuals with comorbid psychiatric illnesses and/or addiction. XIV International AIDS Conference. Barcelona, Spain 2002.
- Shata MT, Anthony DD, Carlson NL, et al. Characterization of the immune response against hepatitis C infection in recovered, and chronically infected chimpanzees. J Viral Hepat 9(6):400-10. 2002.
- Shehab TM, Sonnad SS, Gebremariam A, et al. Knowledge of hepatitis C screening and management by internal medicine residents: trends over 2 years. Am J Gastroenterol 97(5): 1216-22. 2002.
- Shehab TM, Sonnad SS, Jeffries M, et al. Current practice patterns of primary care physicians in the management of patients with hepatitis C. Hepatology 30(9):794-800. 1999.
- Shehab TM, Sonnad SS, Lok AS. Management of hepatitis C patients by primary care physicians in the USA: results of a national survey. J Viral Hepat 8 (5): 377-83. 2001.
- Sherman KE, Rouster SD, Chung RT, et al. Hepatitis C prevalence among patients infected with human immunodeficiency virus: a cross-sectional analysis of the US adult AIDS clinical trials group. Clin Infect Dis 34:831-837. 2002.
- Shi ST, Polyak SJ, Tu H, et al. Hepatitis C virus NS5A colocalizes with the core protein on lipid droplets and interacts with apolipoproteins. Virology 292(2):198-210. 2002.
- Shimizu YK, Feinstone SM, Kohara M, et al. Hepatitis C virus: detection of intracellular virus particles by electron microscopy. Hepatology 23(2):205-9. 1996.
- Shirota Y, Luo H, Qin W, et al. Hepatitis C virus (HCV) NS5A binds RNA-dependent RNA polymerase (RdRP) NS5B and modulates RNA-dependent RNA polymerase activity. J Biol Chem 277(13):11149-55. 2002.
- Solmi L, Primerano AM, Gandolfini L. Ultrasound follow-up of patients at risk for hepatocellular carcinoma: results of a prospective study on 360 cases. Am J Gastroenterol 91 (6):1189-94. 1996.
- Soyer P, Debroucker F, Lacheheb D, et al. [Ultrasound-guided biopsy in focal lesions of the liver. Report on an automated biopsy system — Article in French]. J Radiol 74(4):215-9. 1993.
- Spahn CM, Kieft JS, Grassucci RA, et al. Hepatitis C virus IRES RNA-induced changes in the conformation of the 40s ribosomal subunit. Science 291(5510):1959-62. 2001.
- Staples CT, Rimland D, Dudas D. Hepatitis C in the HIV (human immunodeficiency virus) Atlanta V.A. (Veterans affairs medical center) cohort study (HAVACS): the effect of co-infection on survival. Clin Infect Dis 29(1):150-4. 1999.
- Substance Abuse and Mental Health Services Administration, Office of Applied Studies. National Household Survey on Drug Abuse, 2000 and 2001.
- Sulkowski MS, Moore RD, Mehta SH, et al. Hepatitis C and progression of HIV disease. JAMA 288(2):199-206. 2002.
- Sylvestre DL. Treating hepatitis C in methadone maintenance patients: an interim analysis. Drug Alcohol Depend 67 (2): 117-23. 2002.
- Telfer P, Sabin C, Devereux H, et al. The progression of HCV-associated liver disease in a cohort of haemophiliac patients. Br J of Haematology 87:555-61. 1994.
- Takamizawa A, Mori C, Fuke I, et al. Structure and organization of the hepatitis C virus genome isolated from human carriers. J Virol 65(3):1105-13. 1991.
- Takikawa S, Ishii K, Aizaki H, et al. Cell fusion activity of hepatitis C virus envelope proteins. J Virol 74(11):5066-74. 2000.
- Tan SL, Pause A, Shi Y, Sonenberg N. Hepatitis C therapeutics: current status and emerging strategies. Nat Rev Drug Discov 1(11):867-81. 2002.
- Taylor DR. Hepatitis C virus and interferon resistance: it’s more than just PKR. Hepatology 33(6):1547-9. 2001.
- Tellinghuisen TL, Rice CM. Interaction between hepatitis C virus proteins and host cell factors. Curr Opin Microbiol 5(4):419-27. 2002.
- Thomas DL, Vlahov D, Solomon L, et al. Correlates of hepatitis C virus infections among injecting drug users. Medicine (Baltimore) 74(4):212-20. 1995a.
- Thomas DL, Villano SA, Riester KA, et al. Perinatal transmission of hepatitis C from human immunodeficiency virus type-1 infected mothers. J Infect Dis 177:1480-88. 1998.
- Thomas DL, Astemborski J, Rai RM, et al. The natural history of hepatitis C infection. JAMA 284(4):456. 2000a.
- Thomas DL. Hepatitis C and human immunodeficiency virus infection. Hepatology 36 (5): S201-209. 2002.
- Thomson M, Nascimbeni M, Havert MB, et al. The clearance of hepatitis C virus infection in chimpanzees may not necessarily correlate with the appearance of acquired immunity. J Virol 77(2):862-70. 2003.
- Thorpe LE, Ouellet LJ, Hershow R, et al. Risk of hepatitis C virus infection among young adult injection drug users who share injection equipment. Am J Epidemio 155(7):645-53. 2002.
- Tokars JL, Frank M, Alter, MJ, et al. National surveillance of dialysis-associated diseases in the United States, 2000. Semin Dial 15(3)162-71. 2002.
- Tokushige K, Tsuchiya N, Hasegawa K, et al. Influence of TNF gene polymorphism and HLA-DRB1 haplotype in Japanese patients with chronic liver disease caused by HCV. Am J Gastroenterol 98(1):160-6. 2003.
- Tseng CT, Klimpel GR. Binding of the hepatitis C virus envelope protein E2 to CD81 inhibits natural killer cell functions. J Exp Med 195(1):43-9. 2002.
- Tseng CT, Miskovsky E, Houghton M, Klimpel GR. Characterization of liver TCR?d+ T cells obtained from individuals chronically infected with hepatitis C virus (HCV): evidence for these T cells playing a role in the liver pathology associated with HCV infections. Hepatology 33(5):1312-20. 2001.
- Tsutsumi T, Suzuki T, Shimoike T, et al. Interaction of hepatitis C virus core protein with retinoid X receptor alpha modulates its transcriptional activity. Hepatology 35(4):937-46. 2002.
- United States Public Health Service, Guidelines for the Use of Antiretroviral Agents in HIV-Infected Adults and Adolescents 2002. www.aidsinfo.nih.gov/guidelines/adult/html_adult_02-04-02.html. 2002.
- United States Public Health Service/Infectious Diseases Society of America. Guidelines for preventing opportunistic infections among HIV-infected persons — 2002. MMWR Recommendations and Reports 51 (RR-8). www.cdc.gov/mmwr/preview/mmwrhtml/rr5108a1.htm. 2002.
- Vento S, Garfano F, Renzini C, et al. Fulminant hepatitis associated with hepatitis A virus superinfection in patients with chronic hepatitis C. N Engl J Med 338(5):286-90. 1998.
- Vento, S. Fulminant hepatitis associated with hepatitis A virus superinfection in patients with chronic hepatitis C. J Viral Hepatol 7 (Suppl 1):7-8. 2000.
- Vidal-Trecan GM, Varescon-Pousson I, Gagniere B, et al. Association between first injection risk behaviors and hepatitis C seropositivity among injecting drug users. Ann Med Interne (Paris) 153(4):219-25. 2002.
- Villano SA, Vlahov D, Nelson KE, et al. Persistence of viremia and the importance of long-term follow-up after acute hepatitis C infection. Hepatology 29(3):908-14. 1999.
- Walewski JL, Keller TR, Stump DD, Branch AD. Evidence for a new hepatitis C virus antigen encoded in an overlapping reading frame. RNA 7(5):710-21. 2001.
- Wiese M, Berr F, Lafrenz M, et al. Low frequency of cirrhosis in a hepatitis C (genotype 1b) single-source outbreak in Germany: a 20-year multicenter study. Hepatology 32(1):92-6. 2000.
- WHO Consultation organized in collaboration with the Viral Hepatitis Prevention Board. Global surveillance and control of hepatitis C. J Viral Hepatitis; 6: 35-47. 1999.
- Wunschmann S, Medh JD, Klinzmann D, et al. Characterization of hepatitis C virus (HCV) and HCV E2 interactions with CD81 and the low-density lipoprotein receptor. J Virol 74(21):10055-62. 2000.
- Yanagi M, Purcell RH, Emerson SU, Bukh J. Transcripts from a single full-length cDNA clone of hepatitis C virus are infectious when directly transfected into the liver of a chimpanzee. Proc Natl Acad Sci USA 94(16):8738-43. 1997.
- Yao N, Hesson T, Cable M, et al. Structure of the hepatitis C virus RNA helicase domain. Nat Struct Biol 4(6):463-7. 1997.
- Yee LJ, Tang J, Gibson AW, et al. Interleukin 10 polymorphisms as predictors of sustained response in antiviral therapy for chronic hepatitis C infection. Hepatology 33(3):708-12. 2001.
- Yeung LTF, King SM, Roberts EA. (concise review) Mother-to-infant transmission of hepatitis C virus. Hepatology 34(2):223-227. 2001.
- Yoshida H, Kato N, Shiratori Y, et al. Hepatitis C virus core protein activates nuclear factor kappa B-dependent signaling through tumor necrosis factor receptor-associated factor. J Biol Chem 276(19):16399-405. 2001.
- Zdilar D, Franco-Bronson K, Buchler N, et al. Hepatitis C, interferon alfa, and depression. Hepatology 31(6):1207-1211. 2000.
Tracy Swan is the author of TAG’s upcoming Hepatitis C/HIV Coinfection Report, Version 2.0. She is delighted to have the opportunity to bring together her experience in direct service, advocacy, education, program development, policy, and research for TAG. She created a Hepatitis C Program at Cambridge Health Alliance for active and recovering drug users, developed a curriculum for the Massachusetts Department of Public Health, provided HIV and HCV treatment education, and is a member of the Adult AIDS Clinical Trials Group’s Community Constituency Group, as representative to the Liver Disease Subcommittee and the Immunology Research Agenda Committee.
The Treatment Action Group (TAG) fights to find a cure for AIDS and to ensure that all people living with HIV receive the necessary treatment, care, and information they need to save their lives. TAG focuses on the AIDS research effort, both public and private, the drug development process, and our nation’s health care delivery systems. We meet with researchers, industry, and government officials, and resort when necessary to acts of civil disobedience, or to acts of Congress. We strive to develop the scientific and political expertise needed to transform policy. TAG is committed to working for and with all communities affected by HIV.
Contact us: TAG welcomes feedback and comments on this report and inquiries on our work.
TAG can be reached at: Treatment Action Group 611 Broadway, Suite 612 New York, NY 10012 USA; 212.253.7922 p; 212.253.7923 f; Web site: www.treatmentactiongroup.org;
Suggested citation: T Swan. Research and Policy Recommendations for Hepatitis C Virus (HCV)/HIV Coinfection: Critical Issues from TAG’s forthcoming HCV/HIV Coinfection Report, version 2.0. Treatment Action Group, New York, USA, February 2003.
Acknowledgments: Thanks to the board, staff, consultants, volunteers, and generous donors of TAG. Without you, this report would not have been possible.
Special thanks go to Lei Chou for his flawless graphic design; Andrea Benzacar for her meticulous editing; Mark Harrington and Bob Huff for their collaboration and editorial insight; Daniel Raymond for his contributions to the recommendations for basic research and drug development, and for his editorial support; and to my medical editor, Kenneth E. Sherman, for his expert editorial assistance. Many thanks to each of you for your generosity with your time and your talents.
To Will Berger, for keeping the curtains on fire.
To the authors of The Hepatitis Report, version 1.0, Michael Marco and Jeffery Schouten, who paved the way for this report with their work.
To the staff of the New York Academy of Medicine Library’s Document Delivery Department: Rochelle Castillo, Ruth Dizon, William Landers, Walter Linton, William Spivey, Anthony Taylor, and Elena Vassilkioti, with gratitude and appreciation for your help.
Many individuals contributed to this project: Miriam Alter, George Bishopric, Laurie Carlson, Ray Chung, Dan Church, Allan Clear, Jen Curry, Peter Ferenci, Alan Franciscus, James Goedert, Judy Greenspan, Donald Grove, Robert Heimer, Lisa Hirschhorn, Brian Klein, James Learned, Jules Levin, Greg Lucas, Donny Moss, Marion Peters, Sharon Phillips, Thierry Poynard, Margaret Ragni, Michelle Roland, Leonard Seeff, Mark Sulkowski, Andi Thomas, David Thomas, Francesca Torriani, Annemarie Wasley, Laura Whitehorn, Teresa Wright, and Nizar Zein. Thank you!
This report is dedicated to Shari Brenner.
