March 2014
By Mike Frick
Introduction
The goal of eliminating tuberculosis (TB) as a public health threat in the United States is under threat. In 1989, the U.S. government committed to ending the TB epidemic through the formation of a national TB elimination plan.1 Since then, the heart of the U.S. strategy for domestic TB elimination has progressed through a combination of visionary research linked to bold implementation. As a result of this two-pronged strategy, there were only 9,951 new domestic cases of TB in 2012, the lowest level since reporting began in 1953.2 Yet falling funding for TB research threatens to roll back the hard-won achievements of the last two decades.
Since TB is an airborne infectious disease, the stubborn persistence of the global TB epidemic (which caused 1.3 million deaths and made 8.6 million people fall sick in 20123) means that the U.S. government will need to maintain substantial investments in TB research through the next decade. Individuals born outside of the United States now account for the majority of TB cases reported by state TB programs each year; consequently, eliminating TB domestically hinges on reducing the burden of TB in high-prevalence countries.4
To achieve this, the U.S. government must increase its support for research to develop new tools to fight TB. The most common TB diagnostic test is over 100 years old, and the only available TB vaccine was introduced in 1921 and offers limited protection to adolescents and adults. Most alarmingly, research over the last 40 years has produced only two new drugs to treat TB. This pales in comparison with the speed of research to tackle two closely related diseases: HIV and hepatitis C. Advances in drug discovery have transformed hepatitis C, once a chronic condition, into a curable infection, and the U.S. Food and Drug Administration has approved 36 drugs or combinations of drugs to treat HIV since 1987.5 The development of drug-resistant TB has now outpaced the speed of drug discovery and approval; in 2012, the World Health Organization estimated that there were over 600,000 cases of drug-resistant TB worldwide.6
This policy brief reviews U.S. government (USG) investments in TB research and development (R&D) from 2009 to 2012 and discusses the chilling impact that lower USG spending levels will have on research to develop the new diagnostic tests, drugs, and vaccines needed to realize the goal of TB elimination at home and abroad. These data build on a series of annual surveys conducted by Treatment Action Group tracking global spending on TB R&D in six areas of research: basic science, diagnostics, drugs, vaccines, operational research, and infrastructure/unspecified projects.7
Flatlined: U.S. TB R&D Funding, 2009–2012
Between 2009 and 2012, USG TB R&D funding totaled $1.03 billion out of the $2.53 billion spent globally, or 40.5% of total spending during this period. Figure 1 illustrates the USG share of global TB R&D spending from 2009 to 2012. These investment levels fall far below the $2.00 billion the Stop TB Partnership estimates the world must spend on TB R&D each year in order to eliminate the disease.8 Consequently, every category of research, from drugs to diagnostics to vaccines, suffers from major funding shortfalls. With only $627.4 million spent on TB R&D globally in 2012, even small drops in spending year to year pose serious setbacks to progress and scientific capacity.
Figure 1. USG investments in TB R&D as a proportion of the global total, 2009–2012
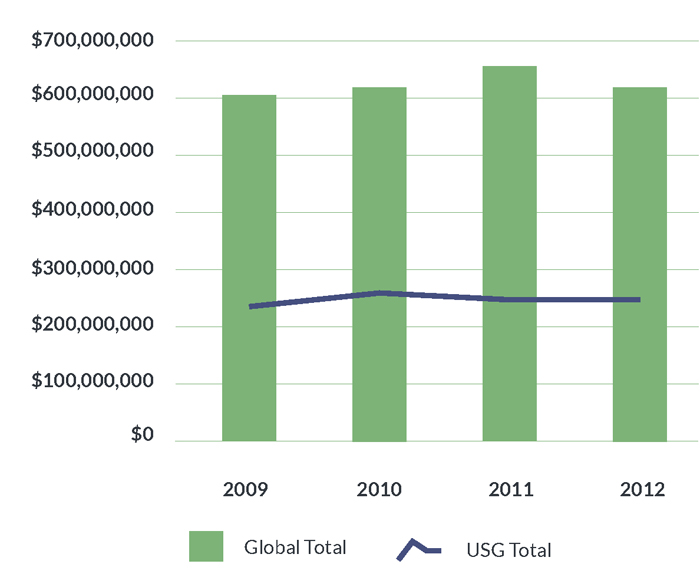
Funding for TB research from 2009 to 2012 weathered substantial volatility. The American Recovery and Reinvestment Act (ARRA) led to watershed investment levels in 2010 due to an infusion of stimulus funds supporting biomedical research. This raised USG TB R&D funding from $255.4 million in 2009 to $268.1 million in 2010. The expiration of the ARRA in 2011 produced a steep drop in spending to $248.1 million followed by a slight recovery to $256.5 million in 2012. Public- sector spending on TB R&D suffered further hits in 2013 when, as a result of sequestration, the U.S. Congress mandated across-the-board cuts to federal agency budgets. In consequence, the National Institutes of Health (NIH) reduced its overall budget by 5% and the Centers for Disease Control and Prevention (CDC) cut funding for the Tuberculosis Trials Consortium (TBTC), a hub of programmatically relevant TB research, by 13%, resulting in the closure of three clinical trials sites.
Underlying this swing from stimulus to sequestration, from boom to bust, is a powerful current, purchasing power parity, that has further eroded the real dollar value of annual research spending. Below the highly visible evaporation of funds under sequestration, high biomedical R&D price inflation has steadily reduced the spending power of USG investments in TB R&D. The Biomedical Research and Development Price Index (BRDPI) calculates how much NIH expenditures must change each year to maintain purchasing power.9 According to this index, the cost of NIH activities increased by 7.4% between fiscal year (FY) 2009 and FY 2012, yet the NIH’s overall R&D budget sustained decreases of close to 20% over the past decade due to inflation. Consequently, while the nominal amount spent on TB R&D in 2012 may suggest partial recovery, when adjusted for inflation, these modest gains are not gains at all and actually extend a decade-long process of declining funding for biomedical research.10
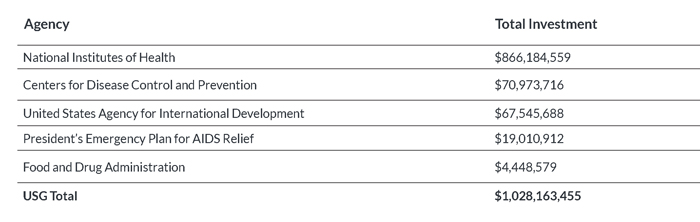
Table 1: TB R&D Investments by USG Agencies, 2009–2012
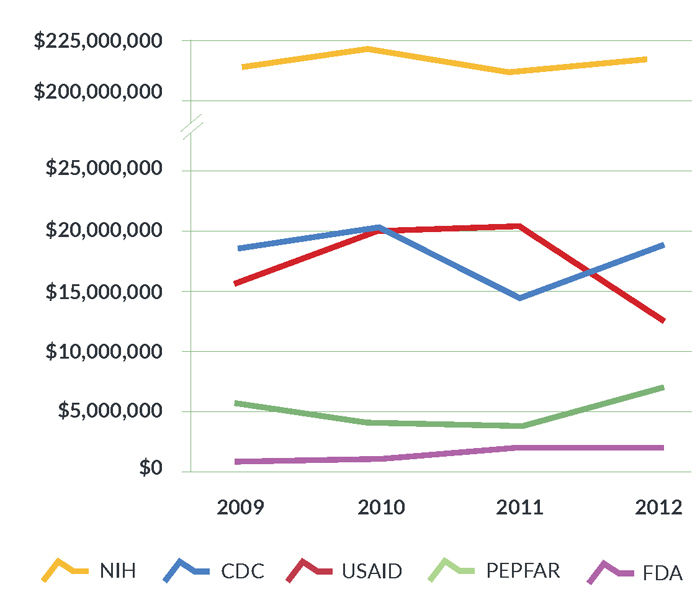
TB R&D Funding Trends within USG Agencies, 2009–2012
- National Institutes of Health (NIH): The NIH contributed 34.2% of global spending on TB R&D from 2009 to 2012, making it the leading funder of TB R&D worldwide. The NIH is also the largest public-sector funder in nearly every category of research, meaning that its contributions to TB R&D cannot be replaced by other agencies or sectors. The NIH plays a unique role in facilitating “translational science,” or helping industry and academic partners turn scientific knowledge into new products for improved TB prevention and care. The apex of NIH TB R&D funding occurred in 2010 under the ARRA, when NIH institutes and centers invested $224.1 million. Since then, funding levels have fluctuated from $208.7 million in 2011 to $217.6 million in 2012. However, these nominal gains are offset when adjusted for inflation. In 2013, sequestration-related cuts further reduced the NIH’s overall budget by $1.55 billion, and FY 2014 appropriations have left the NIH budget below its pre-sequestration level.11
- Centers for Disease Control and Prevention (CDC): The CDC’s TB research portfolio concentrates on drug development and diagnostic evaluation through the TBTC and the TB Epidemiologic Studies Consortium (TBESC). The CDC’s TB research is distinguished by its programmatic relevance, and TBTC studies have paid enormous dividends to public health in the United States and around the world. The TBTC has conducted critical research to develop shorter treatment regimens for curing and preventing TB, and has done so on a shoestring budget. Expiration of the ARRA stimulus dropped CDC spending on TB research by 28.6%, from $19.9 million in 2010 to $14.2 million in 2011. Funding recovered to $18.5 million in 2012, but this reprieve did not last. Sequestration forced the CDC to cut the TBTC’s FY 2013 budget by 13%. Further cuts would jeopardize the TBTC’s plan to conduct a phase III study that, if successful, would shorten the length of TB treatment from six to four months. This potentially groundbreaking study would save millions of dollars in treatment costs each year and greatly reduce the burden of TB treatment on patients, but cannot proceed without renewed funding.
- United States Agency for International Development (USAID): USAID funding for TB R&D focuses on operational research and diagnostic development. Between 2009 and 2010, USAID TB R&D investments increased 28.2%, from $15.4 million to $19.8 million, and then held steady in 2011. These gains were erased in 2012 when funding plummeted by 39.6% to $12.2 million. USAID funding for diagnostics research fell from $5.0 million in 2011 to $2.9 million in 2012, and funding for operational research fell from $9.1 million to $1.7 million. Increasing support for USAID’s TB program will be essential to ensuring that scientific advancements made by other USG agencies are translated into practice abroad, where the TB epidemic is most severe.
- President’s Emergency Plan for AIDS Relief (PEPFAR): PEPFAR supports TB R&D through operational research, spending for which fell 31.3% from $5.3 million in 2009 to $3.6 million in 2010 before dipping another 4.3% in 2011. PEPFAR spending on TB operational research bounced back in 2012 to reach a high of $6.6 million. Efforts to improve the effectiveness of TB programs in PEPFAR-supported countries in accordance with the PEPFAR Blueprint: Creating an AIDS-Free Generation drove this increase. In particular, PEPFAR has invested heavily in operational research related to the scale-up and implementation of GeneXpert, a system that can diagnose TB and resistance to the first-line TB drug rifampicin within two hours.
- Food and Drug Administration (FDA): The FDA supports TB research through its Orphan Products Grants Program, which has funded U.S. universities to study TB drugs including rifapentine. The FDA also supports two product-development partnerships headquartered in the United States: the Global Alliance for TB Drug Development, and Aeras. The Global Alliance conducts research to develop safer, faster-acting new drugs to treat TB, and Aeras is working to develop new, more efficacious TB vaccines. FDA funding increased from $400,000 in 2009 to $1.6 million in 2012 as it started supporting Aeras and the Global Alliance, although the FDA’s investments remain far below those of its sister USG institutions.
Figure 2. Trends in TB R&D investments among USG agencies, 2009–2012
Messages for Policy Makers
As the world’s leading investor in TB R&D, the U.S. government has greatly advanced the development of new tools to better prevent, diagnose, and treat TB, saving millions of lives and improving the global standard of care. Yet funding stagnation since 2009, compounded by the weakened purchasing power of research dollars and the shortsighted slashes to public budgets under sequestration, threaten to stall progress at a critical juncture on the road to TB elimination.
In the short term, promising new research proposals will go uninvestigated as unstable funding creates uncertainty about the availability of funds. Lower funding levels mean fewer opportunities for young investigators to build their scientific careers in TB research. TB research networks based in the United States have already closed clinical trials sites, resulting in lost jobs for site staff and lost scientific expertise as research teams redirect to other fields.
Developing the new diagnostics, drugs, and vaccines urgently needed to fight TB and overcome growing drugresistance depends on commitments to TB research made by the U.S. government today. To end the TB epidemic in the United States and abroad, members of Congress must:
- Increase multiyear funding for TB R&D activities by federal agencies and peg these increases to the Biomedical Research and Development Price Index. USG funding for TB R&D is not replaceable by either other governments or the private sector. Since 2011, two pharmaceutical companies, Pfizer and AstraZeneca, have left the field entirely, while other drug companies have reduced their investments in TB research.12 Other governments (particularly those of middleincome countries) can and should invest more in TB research, but cannot match the spending power or scientific capacity of U.S. public agencies.
- Reauthorize the Comprehensive Tuberculosis Elimination Act, sponsored by U.S. Representative Gene Greene (D-TX), which sanctions the important roles of the NIH, CDC, and FDA in TB R&D. The Act updates NIH TB research priorities to respond to recent trends in the global and domestic epidemiology of TB. These updated priorities include research on pediatric TB (a traditionally neglected area of study) and research to respond to TB infection, now the focus of TB elimination work in the United States. Passage of the Act will support broader efforts to combat drug resistance, the subject of a 2013 CDC threat report, which warned that the United States may soon enter a “post-antibiotic era” without reinvigorated research against infectious diseases like TB.13
- Require increased reporting on progress against TB within the USG’s broader global health initiatives. As the leading killer of people with HIV, TB should occupy a larger focus of the USG’s response to the global AIDS epidemic through support for PEPFAR and the Global Fund to Fight AIDS, TB and Malaria (the Global Fund). The inclusion of TB indicators in new PEPFAR reporting requirements is a positive step forward and should be maintained. As part of its pledge to support the Global Fund’s replenishment, the USG should ask the Global Fund to ensure that recipient countries spend 5–10% of grants on operational research, including research related to TB/HIV coinfection, in line with the Global Fund’s own recommendation to grantees.
Endnotes
- Dowdle WR; Centers for Disease Control (CDC). A strategic plan for the elimination of tuberculosis in the United States. MMWR Morb Mortal Wkly Rep. 1989 Apr 21;38 Suppl 3:1–25.
Available from: http://www.cdc.gov/mmwr/preview/mmwrhtml/00001375.htm. (Accessed 2014 March 13) - Centers for Disease Control and Prevention (U.S.). Trends in tuberculosis— United States, 2012. MMWR Morb Mortal Wkly Rep. 2013 Mar 22;62(11): 201–05.
Available from: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6211a2.htm. (Accessed 2014 March 1) - World Health Organization. Global tuberculosis report 2013. Geneva: World Health Organization; 2013.
Available from: http://www.who.int/tb/publications/global_report/en. (Accessed 2014 March 1) - Starke JR, Cruz AT. The global nature of childhood tuberculosis. Pediatrics. 2014 Mar;133(3):e725-7. doi: 10.1542/peds.2013-4139.
- Clayden P, Harrington M. Seven ways to speed up the pipeline. In: Clayden P, Harrington M, Swan T, et al.; i-Base/Treatment Action Group. 2013 pipeline report: drugs, diagnostics, vaccines, preventive technologies, research toward a cure, and immune-based and gene therapies in development. Edited by Andrea Benzacar. New York: Treatment Action Group; 2013. p. 1–25.
- World Health Organization. Global tuberculosis report 2013.
- Frick M, Jimenez-Levi E. 2013 report on tuberculosis research funding trends, 2005–2012. Edited by Mark Harrington. New York: Treatment Action Group; 2013.
Available from: https://www.treatmentactiongroup.org/tbrd2013. (Accessed 2014 March 1) - Stop TB Partnership. The global plan to stop TB: 2011–2015. Geneva: World Health Organization; 2011.
Available from: http://www.stoptb.org/assets/documents/global/plan/TB_GlobalPlanToStopTB2011-2015.pdf. (Accessed 2014 March 1) - National Institutes of Health (U.S.). Biomedical research and development price index (BRDPI). Available from: http://officeofbudget.od.nih.gov/gbiPriceIndexes.html. (Accessed 2014 March 1)
- AmfAR and Treatment Action Group. The costs of flat funding for biomedical research. August 2013.
Available from: http://www.amfar.org/uploadedFiles/_amfarorg/On_the_Hill/Issue%20Brief%20-%20The%20Costs%20of%20Flat%20Funding%20NIH%20Research_080513.pdf. (Accessed 2014 March 1) - Fiscal year 2014 budget request. Testimony of Francis Collins before the Senate Subcommittee on Labor, Health and Human Services and Education Appropriations Committee. 2013 May 15.
Available from: http://www.nih.gov/about/director/budgetrequest/fy2014testimony.htm. (Accessed 2014 March 1) - Frick M, Jimenez-Levi E. 2013 report on tuberculosis research funding trends.
- Centers for Disease Control and Prevention (U.S.). Antibiotic resistance threats in the United States, 2013. Atlanta, GA: Centers for Disease Control and Prevention; 2013.
Available from: http://www.cdc.gov/drugresistance/threat-report-2013. (Accessed 2014 March 1)