December 2012
By Tracy Swan
It is difficult to be anything other than dazzled by astounding cure rates of up to 100% from a multitude of interferon-free hepatitis C virus (HCV) clinical trials presented at the American Association for the Study of Liver Diseases (AASLD) meeting in November 2012. Proof of concept has been established: hepatitis C, a disease that claims more than 350,000 lives annually, can be cured with three months of oral antiviral drugs.
These incredible advances bear scrutiny, since most of these interferon-free trials enrolled people with minimal liver disease—many of whom were being treated for the first time. Information about safety, efficacy, and tolerability of interferon-free regimens is needed in other groups, such as people coinfected with HIV, liver transplant candidates and recipients, and people with cirrhosis (especially those who are treatment-experienced)—in other words, people with the greatest immediate need of a safe and highly effective cure.
When Is a Cure Really a Cure?
The term sustained virologic response (SVR) is used when the hepatitis C viral load (also called HCV RNA) remains undetectable after completing HCV treatment; it indicates that hepatitis C has been cured. SVR has been proven to lower the risk for liver-related illness and death, although people with pretreatment cirrhosis should be monitored regularly, since they are still at risk for liver cancer.
With pegylated interferon and ribavirin, a person was considered cured when HCV RNA became undetectable during treatment and remained undetectable for 24 weeks after completing therapy (known as SVR-24). Recently, regulators at the U.S. Food and Drug Administration (FDA) revised this time point from SVR-24 to SVR-12, since most posttreatment relapses (when HCV RNA becomes detectable after treatment completion) occur within 12 weeks. Thus, SVR-12 became the new primary outcome for clinical trials studying peginterferon-based regimens.
The hunger for information about cure rates from interferon-free regimens has led to earlier reporting of results; SVR-4 (undetectable HCV RNA 4 weeks after finishing treatment) is now commonly used. But with interferon-free regimens, SVR-4 does not always predict SVR-12, and SVR-12 does not always predict SVR-24. In fact, there have been two late relapses between 24 and 48 weeks after treatment with an interferon-free regimen: one in Abbott’s PILOT trial, and one in Boehringer Ingelheim’s SOUND-C2 trial. In both cases, treatment consisted of an HCV protease inhibitor, a non-nucleoside polymerase inhibitor, and ribavirin (RBV)1,2 Although SVR-12 and SVR-24 are primary outcomes for interferon-free trials, monitoring for late relapse will continue.
Genotype 1
Cure rates for interferon-containing and interferon-free regimens have skyrocketed in people with HCV genotype 1, although in treatment-naive people certain factors such as HCV subtype (1a vs. 1b), IL28B genotype (CC vs. non-CC), and extent of liver damage (advanced vs. mild-to-moderate) may impair response to treatment (see table 1).
Table 1. Interferon-Free Regimens in HCV Genotype 1, Treatment-Naive
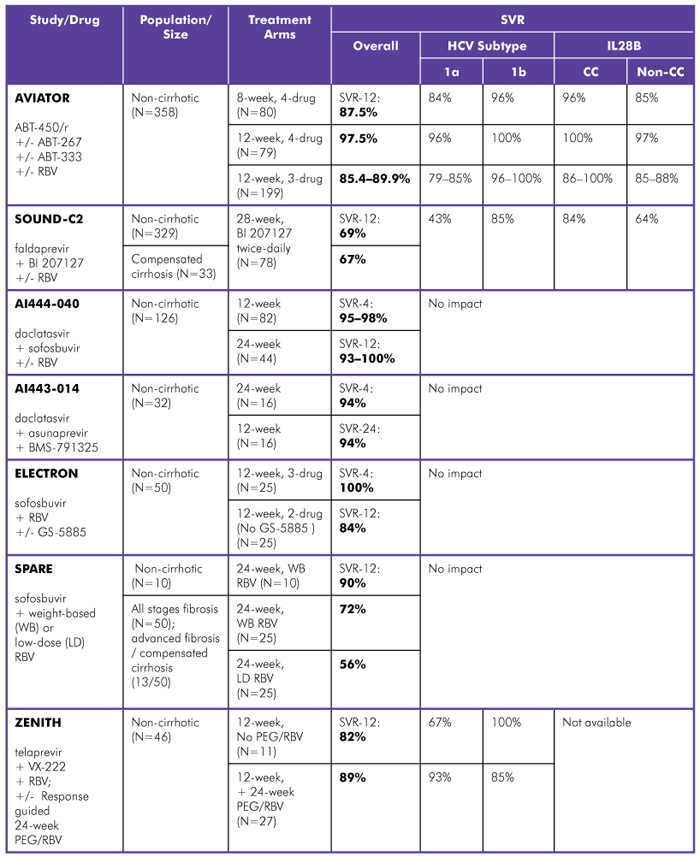
Study Designs:
AVIATOR (Abbott Laboratories): Phase IIb; 3- or 4-drug combinations with ABT-450/r (boosted protease inhibitor), ABT-267 (NS5a inhibitor), ABT-333 (non-nucleoside polymerase inhibitor), and RBV; treated for 8, 12, or 24 weeks. SVR-12 data reported.
SOUND-C2 (Boehringer Ingelheim): Phase IIb; faldaprevir (protease inhibitor) with BI 207127 (non-nucleoside polymerase inhibitor), with or without RBV; treated for 16, 28, or 40 weeks. SVR-12 data reported.
AI444-040 (Bristol-Myers Squibb/Gilead Sciences): Phase IIa; daclatasvir (NS5a inhibitor) with sofosbuvir (nucleotide polymerase inhibitor), with or without RBV; treated for 12 or 24 weeks. SVR-12 data reported.
AI443-014 (Bristol-Myers Squibb): Phase II; daclatasvir (NS5a inhibitor) with asunaprevir (protease inhibitor) and BMS-791325 (non-nucleoside polymerase inhibitor); treated for 12 or 24 weeks. SVR-4 and SVR-24 data reported. Comment: Gilead is developing an in-house co-formulation of sofosbuvir and GS-5885, their NS5a inhibitor, rather than continuing codevelopment with BMS.
ELECTRON (Gilead Sciences): Phase II; sofosbuvir (nucleotide polymerase inhibitor) with RBV, with or without GS-5885 (NS5a inhibitor); treated for 12 weeks. SVR-12 data reported.
SPARE (Gilead Sciences/NIH Partnership): Phase II; mostly African American, IL28B non-CC, and non-cirrhotic; sofosbuvir (nucleotide polymerase inhibitor) with weight-based dosing (WBD) of RBV or low-dose RBV (600 mg); treated for 24 weeks. SVR-12 data reported. Comment: Prior to week 12, 8 out of 60 participants discontinued treatment.
ZENITH (Vertex Pharmaceuticals): Phase II; telaprevir (protease inhibitor) with VX-222 (non-nucleoside polymerase inhibitor) and RBV: Response-guided treatment for 12 weeks, plus 24 weeks of PEG/RBV if HCV RNA detectable at week 2 or 8. SVR-12 data reported.
Sources:
Everson GT, Sims KD, Rodriguez-Torres M, et al. An interferon-free, ribavirin-free 12-week regimen of daclatasvir (DCV), asunaprevir (ASV), and BMS-791325 yielded SVR4 of 94% in treatment-naive patients with genotype (GT) 1 chronic hepatitis C virus (HCV) infection (Abstract LB-3). Paper presented at: 63rd Annual Meeting of the American Association for the Study of Liver Diseases (AASLD); 2012 November 9–13; Boston, MA.
Gane EJ, Stedman CJ, Hyland RH, et al. Once daily sofosbuvir (GS-7977) regimens in HCV genotype 1–3: The ELECTRON trial (Abstract 229). Paper presented at: 63rd Annual Meeting of the American Association for the Study of Liver Diseases (AASLD); 2012 November 9–13; Boston, MA.
Kowdley KV, Lawitz E, Poordad F, et al. A 12-week interferon-free treatment regimen with ABT-450/r, ABT 267, ABT-333, and ribavirin achieves SVR12 rates (observed data) of 99% in treatment-naive patients and 93% in prior null responders with HCV genotype 1 infection (Abstract LB-1). Paper presented at: 63rd Annual Meeting of the American Association for the Study of Liver Diseases (AASLD); 2012 November 9–13; Boston, MA.
Osinusi A, Heytens L, Lee YJ, et al. High efficacy of GS-7977 in combination with low or full dose ribavirin for 24 weeks in difficult to treat HCV infected genotype 1 patients (Abstract LB-4). Paper presented at: 63rd Annual Meeting of the American Association for the Study of Liver Diseases (AASLD); 2012 November 9–13; Boston, MA.
Soriano V, Gane EJ, Angus P, et al. Efficacy and safety of the interferon-free combination of faldaprevir (BI 201335) + BI 207127 ± ribavirin in treatment-naive patients with HCV GT-1 and compensated liver cirrhosis: results from the SOUND-C2 study A (Abstract 84). Paper presented at: 63rd Annual Meeting of the American Association for the Study of Liver Diseases (AASLD); 2012 November 9–13; Boston, MA.
Sulkowski MS, Gardiner DF, Rodriguez-Torres M, et al.; AI444040 Study Group. High rate of sustained virologic response with the all-oral combination of daclatasvir (NS5a inhibitor) plus sofosbuvir (nucleotide NS5b inhibitor) with or without ribavirin, in treatment-naive patients chronically infected with HCV GT 1, 2, or 3 (Abstract LB-2). Paper presented at: 63rd Annual Meeting of the American Association for the Study of Liver Diseases (AASLD); 2012 November 9–13; Boston, MA.
Sulkowski MS, Jacobson IM, Gane EJ. The safety of telaprevir in the absence of interferon and/or ribavirin: analysis of on-treatment data from the ZENITH trial (Abstract 786). Paper presented at: 63rd Annual Meeting of the American Association for the Study of Liver Diseases (AASLD); 2012 November 9–13; Boston, MA.
Zeuzem S, Soriano V, Asselah T, et al. Interferon (IFN)-free combination treatment with the HCV NS3/4A protease inhibitor faldaprevir (BI 201335) and the non-nucleoside NS5B inhibitor BI 207127 ± ribavirin: final results of SOUND-C2 and predictors of response (Abstract 232). Paper presented at: 63rd Annual Meeting of the American Association for the Study of Liver Diseases (AASLD); 2012 November 9–13; Boston, MA.
AASLD also brought good news for treatment-experienced people with HCV genotype 1 (see table 2). Phase III trials of DAA combinations, in both treatment-naive and treatment-experienced people, with and without peginterferon and/or ribavirin, are ongoing or soon to be launched.
Table 2. Interferon-Free Regimens in HCV Genotype 1, Treatment-Experienced
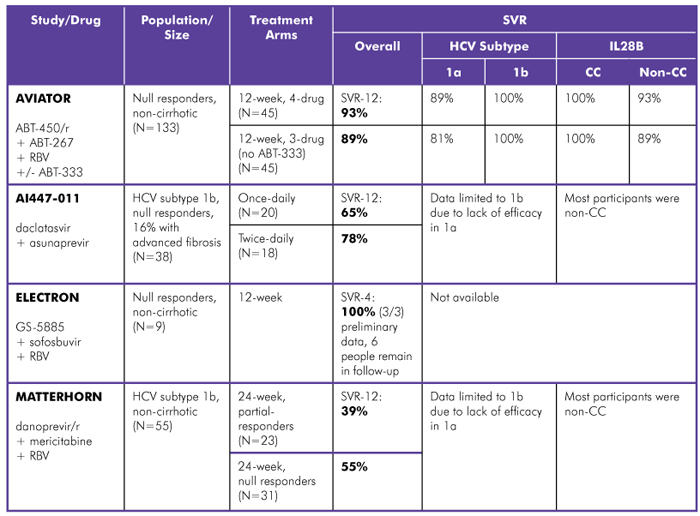
Study Designs:
AVIATOR (Abbott Laboratories): Phase IIb; ABT-450/r (boosted protease inhibitor) with ABT-267 (NS5a inhibitor) and RBV, with or without ABT-333 (non-nucleoside polymerase inhibitor); treated for 12 or 24 weeks. SVR-12 data reported.
AI447-011 (Bristol-Myers Squibb): Phase II; daclatasvir (NS5a inhibitor) with asunaprevir (protease inhibitor), once- or twice-daily; treated for 24 weeks. SVR-12 data reported. Comment: An additional arm in this trial, the 2-drug combination plus RBV, was given to people with HCV genotypes 1a and 1b; due to high rates of viral breakthrough (10/18), peginterferon was added (8/18) and participants are being followed.
ELECTRON (Gilead Sciences): Phase II; GS-5885 (NS5a inhibitor) with sofosbuvir (nucleotide polymerase inhibitor) and RBV; treated for 12 weeks. SVR-4 data reported.
MATTERHORN (Hoffmann-La Roche): Phase II; danoprevir/r (boosted protease inhibitor) with mericitabine (nucleoside polymerase inhibitor) and RBV; treated for 24 weeks. SVR-12 data reported. Comment: HCV subtype 1a participants had high rates of virologic breakthrough during the study; peginterferon was added and data not included; baseline viral loads were higher in partial responders vs. null responders.
Sources:
Feld JJ, Jacobson IM, Jensen DM, et al. Up to 100% SVR4 rates with ritonavir-boosted danoprevir (DNVr),nmericitabine and ribavirin with or without peginterferon alfa-2a (40KD) in genotype1-infected partial and null responders: results from the MATTERHORN study (Abstract 81). Paper presented at: 63rd Annual Meeting of the American Association for the Study of Liver Diseases (AASLD); 2012 November 9–13; Boston, MA.
Gane EJ, Stedman CJ, Hyland RH, et al. Once daily sofosbuvir (GS-7977) plus ribavirin in HCV genotype 1–3: The ELECTRON trial (Abstract 229). Paper presented at: 63rd Annual Meeting of the American Association for the Study of Liver Diseases (AASLD); 2012 November 9–13; Boston, MA.
Kowdley KV, Lawitz E, Poordad F, et al. A 12-week interferon-free treatment regimen with ABT-450/r, ABT 267, ABT-333, and ribavirin achieves SVR12 rates (observed data) of 99% in treatment-naive patients and 93% in prior null responders with HCV genotype 1 infection (Abstract LB-1). Paper presented at: 63rd Annual Meeting of the American Association for the Study of Liver Diseases (AASLD); 2012 November 9–13; Boston, MA.
Lok AS, Gardiner DF, Hézode C, et al. Sustained virologic response in chronic HCV genotype (GT) 1-infected null responders with combination of daclatasvir (DCV; NS5a inhibitor) and asunaprevir (ASV; NS3 inhibitor) with or without peginterferon alfa-2a/ribavirin (PEG/RBV) (Abstract 79). Paper presented at: 63rd Annual Meeting of the American Association for the Study of Liver Diseases (AASLD); 2012 November 9–13; Boston, MA.
Although people with hepatitis C and their medical providers want to dispense with interferon and ribavirin, some people—especially null responders with HCV genotype 1a and IL28B non-CC genotypes—may require one or both drugs plus a combination of direct-acting antivirals (DAAs) for a cure. Therefore, regimens that shorten duration of pegylated interferon and/or ribavirin, or substitute peginterferon lambda (a potentially more tolerable type of interferon) for peginterferon alfa, are moving forward (see table 3).
Table 3. Interferon-Based Regimens in HCV Genotype 1, Treatment-Naive and Treatment-Experienced
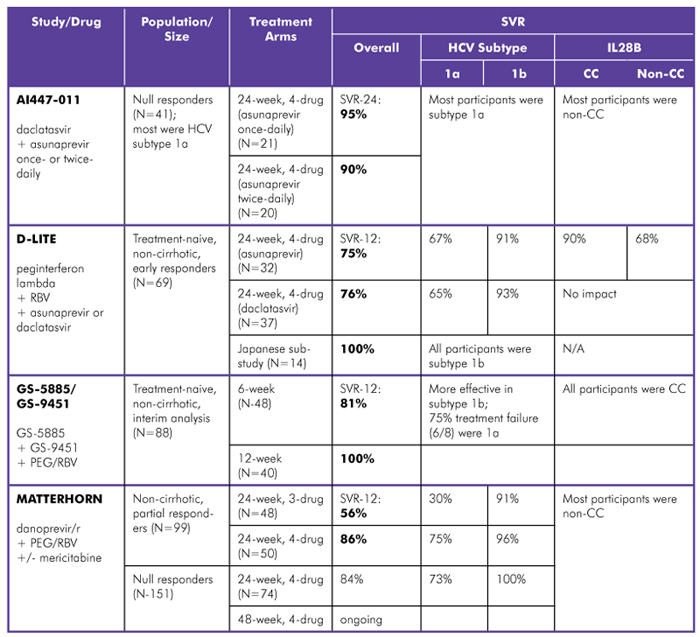
Study Designs:
AI447-011 (Bristol-Myers Squibb): Phase II; daclatasvir (NS5a inhibitor) with peginterferon, RBV, and asunaprevir (protease inhibitor) once- or twice-daily; treated for 24 weeks. SVR-24 data reported. Comment: an additional arm in this trial, the 2-drug combination plus RBV, was given to people with HCV genotypes 1a and 1b; Due to high rates of viral breakthrough (10/18), peginterferon was added (8/18) and participants are being followed.
D-LITE(Bristol-Myers Squibb): Phase IIb; peginterferon lambda and RBV, with asunaprevir (protease inhibitor) or daclatasvir (NS5a inhibitor); response-guided treatment for 24 or 48 weeks. SVR-12 data from early responders, 24-week treatment arm reported. Comment: daclatasvir was more tolerable than asunaprevir.
GS-5885/GS-9451 (Gilead Sciences): Phase II; GS-5885 (NS5a inhibitor) with GS-9451 (protease inhibitor), and peginterferon+RBV; treated for 6 or 12 weeks. SVR-12 interim analysis reported. Comment: data from participants in posttreatment follow-up and people without early response not yet available.
MATTERHORN (Hoffmann-La Roche): Phase II; danoprevir/r (boosted protease inhibitor) with peginterferon+RBV, with or without mericitabine (nucleoside polymerase inhibitor); partial responders treated for 24 weeks, null responders treated for 24 or 48 weeks. SVR-12 data reported. Comment: Although numbers were small, efficacy of 3- or 4-drug regimens did not differ between people with mild-to-moderate liver fibrosis.
Sources:
Feld JJ, Jacobson IM, Jensen DM, et al. Up to 100% SVR4 rates with ritonavir-boosted danoprevir (DNVr), mericitabine and ribavirin with or without peginterferon alfa-2a (40KD) in genotype 1-infected partial and null responders: results from the MATTERHORN study (Abstract 81). Paper presented at: 63rd Annual Meeting of the American Association for the Study of Liver Diseases (AASLD); 2012 November 9–13; Boston, MA.
Izumi N, Lataillade M, Chayama K, et al.; D-LITE Study Team. First report of peginterferon lambda/ribavirin in combination with either daclatasvir or asunaprevir in HCV genotype 1 Japanese patients: early sustained virologic response (SVR4) results from the D-LITE Japanese substudy (Abstract 284). Paper presented at: 63rd Annual Meeting of the American Association for the Study of Liver Diseases (AASLD); 2012 November 9–13; Boston, MA.
Jacobson IM, Jensen DM, Pol S, et al. Safety and efficacy of ritonavir-boosted danoprevir (DNVr), peginterferon alfa-2a (40KD) and ribavirin with or without mericitabine in genotype 1-infected treatment-experienced patients with advanced hepatic fibrosis: the MATTERHORN study (Abstract 82). Paper presented at: 63rd Annual Meeting of the American Association for the Study of Liver Diseases (AASLD); 2012 November 9–13; Boston, MA.
Muir A, Hilson J, Gray T, et al.; EMERGE Study Group. Peginterferon lambda-1a (lamdba) compared with peginterferon alfa-2a (alfa) in treatment naive patients with HCV genotypes 1 or 4: SVR24 results from EMERGE phase 2b (Abstract 214). Paper presented at: 63rd Annual Meeting of the American Association for the Study of Liver Diseases (AASLD); 2012 November 9–13; Boston, MA.
Thompson A, Shiffman M, Rossaro L, et al. Six weeks of an NS5a inhibitor (GS-5885) and a protease inhibitor (GS-9451) plus peginterferon and ribavirin achieves high SVR4 rates in genotype 1 IL28B CC treatment naive hepatitis C virus patients: interim results of a prospective, randomized trial (Abstract 759). Paper presented at: 63rd Annual Meeting of the American Association for the Study of Liver Diseases (AASLD); 2012 November 9–13; Boston, MA.
Vierling JM, Lataillade M, Gane EJ, et al.; D-LITE Study Team. Sustained virologic response (SVR12) in HCV genotype 1 patients receiving peginterferon lambda in combination with ribavirin and either daclatasvir or asunaprevir: interim results from The D-LITE study (Abstract LB-9). Paper presented at: 63rd Annual Meeting of the American Association for the Study of Liver Diseases (AASLD); 2012 November 9–13; Boston, MA.
Genotypes 2 and 3
Results from trials in treatment-naive and treatment-experienced people with HCV genotypes 2 and 3 were presented at AASLD. There was good news for treatment-experienced people, since there is currently no recommended re-treatment option when peginterferon and ribavirin are unsuccessful.
For treatment-naive people with HCV genotypes 2 and 3, interferon-free regimens combining sofosbuvir (a nucleotide polymerase inhibitor) and ribavirin yielded cure rates similar to those achieved with the current standard of care (which is 24 weeks of peginterferon and ribavirin), but duration was shortened to 8 weeks. When daclatasvir (an NS5a inhibitor) was added, SVR increased, but duration doubled from 12 to 24 weeks (see table 4).
In late November 2012, Gilead Sciences issued the somewhat disappointing top-line results from POSITRON, a 278-person, interferon-free phase III trial in HCV genotypes 2 and 3. POSITRON compared 12 weeks of sofosbuvir (a nucleotide polymerase inhibitor) and ribavirin to placebo in treatment-naive, interferon-ineligible, -intolerant, or -unwilling participants. In HCV genotype 2, SVR-12 was close to 100%, but in HCV genotype 3, SVR-12 was only 61%.3
In treatment-naive people with HCV genotypes 2 and 3, interferon-free regimes offer the advantage of improved tolerability and ease of administration. But high prices will make these drugs unappealing to payers without a clear demonstration of improved efficacy and the potential to fill unmet therapeutic needs.
In genotypes 2 and 3, re-treatment regimens—especially for people with HCV genotype 3—should be prioritized by pharmaceutical companies. Sponsors need to develop safe, effective, tolerable, and affordable regimens when no alternatives exist, in addition to improving the existing standard of care.
Table 4. HCV Genotypes 2 and 3, Treatment-Naive and Treatment-Experienced
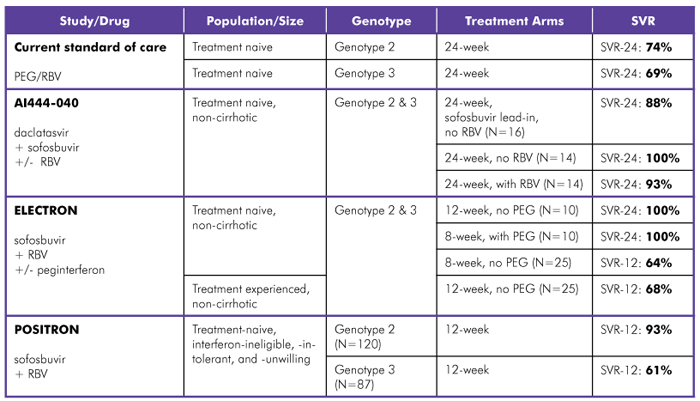
Study Design:
AI444-040 (Bristol-Myers Squibb/Gilead Sciences): Phase IIa; daclatasvir (NS5a inhibitor) with sofosbuvir (nucleotide polymerase inhibitor), with or without RBV; treated for 12 or 24 weeks. SVR-12 data reported.
ELECTRON (Gilead Sciences): Phase II; sofosbuvir (nucleotide polymerase inhibitor) and RBV, with or without peginterferon; treated for 8 or 12 weeks. SVR-12 and SVR-24 data reported. Comment: Regimen most effective with weight-based RBV dosing.
POSITRON (Gilead Sciences): Phase III; sofosbuvir (nucleotide polymerase inhibitor) and RBV; treated for 12 weeks. SVR-12 data reported. Comment: No recommended re-treatment regimen.
Sources:
European Society for the Study of Liver Diseases (EASL). EASL clinical practice guidelines: management of hepatitis C virus infection. June 2011. Available from: http://www.easl.eu/assets/application/files/4a7bd873f9cccbf_file.pdf. (Accessed on November 29 2012)
Gane EJ, Stedman CJ, Hyland RH, et al. Once daily sofosbuvir (GS-7977) regimens in HCV genotype 1–3: the ELECTRON trial (Abstract 229). Paper presented at: 63rd Annual Meeting of the American Association for the Study of Liver Diseases (AASLD); 2012 November 9–13; Boston, MA.
Gilead (Press Release). Gilead announces sustained virologic response rate of 78% from phase 3 study of sofosbuvir for genotype 2/3 hepatitis C infected patients. 2012 November 27. Available from: http://investors.gilead.com/phoenix.zhtml?c=69964&p=irol-newsArticle&ID=1761988&highlight=. (Accessed on 2012 November 28)
Sulkowski MS, Gardiner DF, Rodriguez-Torres M, et al.; AI444040 Study Group. High rate of sustained virologic response with the all-oral combination of daclatasvir (NS5a inhibitor) plus sofosbuvir (nucleotide NS5b inhibitor) with or without ribavirin, in treatment-naive patients chronically infected with HCV GT 1, 2, or 3 (Abstract LB-2). Paper presented at: 63rd Annual Meeting of the American Association for the Study of Liver Diseases (AASLD); 2012 November 9–13; Boston, MA.
Genotypes 4 and 6
For people with HCV genotypes 4 and 6, big news came in a small group of treatment-naive, non-cirrhotic people in the ATOMIC study, treated with 24 weeks of sofosbuvir, peginterferon, and ribavirin. Of the 11 people with HCV genotype 4, 82% had an SVR-12 (two participants, who were responding to treatment, did not return for follow-up visits). In genotype 6, 100% of 5 people had an SVR-12. No relapses were reported between weeks 12 and 24.4
Adding an HCV protease inhibitor to peginterferon and ribavirin boosted SVR and shortened treatment duration for 30 non-cirrhotic, treatment-naive people with HCV genotype 4. The DAUPHINE trial studied 50, 100, or 200 mg of danoprevir/r (a boosted protease inhibitor) plus peginterferon and ribavirin for 24 weeks (with the exception of one arm, where treatment was response-guided; early responders stopped treatment at 12 weeks). Regardless of the danoprevir/r dose, or treatment duration, 97% achieved SVR-24 (one person was lost to follow-up). In the response-guided arm, all seven participants were eligible for 12 week of treatment, and SVR-24 was 100%.5
Peginterferon lambda may be a good option for people with HCV genotype 4, if phase III trials confirm the favorable side effect profile seen in phase II. The EMERGE trial (which compared safety, efficacy, and tolerability of peginterferon alfa and ribavirin vs. peginterferon lambda and ribavirin) included a small group of people with HCV genotype 4 (approximately 18; 12 received peginterferon lambda).6 Although overall efficacy was comparable, lambda was significantly less likely to cause flulike symptoms and laboratory abnormalities such as anemia and neutropenia than was peginterferon alfa-2a.7
HIV/HCV Coinfection
Final results from a phase II trial of telaprevir plus peginterferon and ribavirin in HIV/HCV-coinfected people were presented at AASLD. In this trial, there were no relapses between 12 and 24 weeks posttreatment: 74% of people who received telaprevir-based triple therapy versus 45% of people given peginterferon and ribavirin plus placebo achieved SVR-24. Telaprevir levels were similar in study participants not on antiretroviral therapy and those taking a boosted atazanavir- or efavirenz-based regimen; in turn, antiretroviral concentrations were not significantly altered by telaprevir, confirming results of earlier drug-drug interaction studies.8
Sofosbuvir is already being studied in HIV/HCV-coinfected people with HCV genotypes 2 and 3; a clinical trial comparing 12 versus 24 weeks of sofosbuvir and ribavirin is ongoing.
Results from drug-drug interaction (DDI) studies of sofosbuvir and commonly used antiretroviral agents were presented at AASLD. There were no clinically significant interactions between sofosbuvir and efavirenz, rilpivirine, boosted darunavir, raltegravir, tenofovir, and emtricitabine in healthy volunteers.9 Pharmacokinetics and DDIs may be different in people with hepatitis C—especially those with advanced liver damage—and HIV-positive people compared with healthy volunteers. Nonetheless, the results suggest that these drugs can be coadministered with sofosbuvir, though careful monitoring in clinical trials is still warranted.
Hopefully, the near future will bring more interferon-free trials to HIV/HCV-coinfected people.
Compensated Cirrhosis and Pre- and Posttransplant
Reports of pretransplant cures are encouraging, but severe adverse events and poor tolerability of triple therapy with peginterferon, ribavirin, and an HCV protease inhibitor significantly limit use in more “real-life” people with compensated cirrhosis, especially those with a platelet count of <100,000/mm3 or serum albumin level of <35 g/L.10 In fact, one study conducted at a transplant center reported discontinuation rates among people with compensated cirrhosis of >60%.11 Clearly, DAA combination trials are needed in people with cirrhosis to avert transplantation or treat posttransplant reinfection.
In CRUSH-C, a trial of protease inhibitor–based triple therapy in 103 posttransplant participants, treatment efficacy is promising: 57% of participants had an early response; HCV RNA was undetectable during treatment at week 4 and week 12. But treatment safety and tolerability, as well as management of DDIs with immunosuppressants, complicate treatment in the posttransplant setting. Only 14% discontinued treatment for adverse events, but most participants required dose reductions of peginterferon, ribavirin, or both drugs and/or growth factors, and 48% required blood transfusions. After starting protease inhibitors (90% telaprevir; 10% boceprevir), immunosuppressant dosing was adjusted (cyclosporine was reduced by 75%; tacrolimus by 90%); nonetheless, a third of participants experienced worsening renal function, and graft rejection was noted (during transition off of the protease inhibitor).12
There is a single posttransplant case report of successful interferon- and ribavirin-free treatment of severe, recurrent hepatitis C infection, cured with 24 weeks of daclatasvir and sofosbuvir.13 Other drugs may be combined to create interferon- and ribavirin-free regimens in the posttransplant setting: DDI studies with simeprevir (TMC435, a protease inhibitor currently in phase III) and sofosbuvir did not report clinically significant interactions with immunosuppressants in healthy volunteers.14,15 These glimmers of hope need to materialize into trials and programs providing access to lifesaving drugs—the sooner, the better.
Endnotes
1. Zeuzem S, Soriano V, Asselah T, et al. Interferon (IFN)-free combination treatment with the HCV NS3/4A protease inhibitor faldaprevir (BI 201335) and the non-nucleoside NS5B inhibitor BI 207127± ribavirin: final results of SOUND-C2 and predictors of response (Abstract 232). Paper presented at: 63rd Annual Meeting of the American Association for the Study of Liver Diseases (AASLD); 2012 November 9–13; Boston, MA.
2. Lawitz E, Poordad F, Kowdley KV, et al. A 12-week interferon-free regimen of ABT-450/r, ABT-072 and ribavirin was well tolerated and achieved sustained virologic response in 91% treatment-naïve HCV IL28B CC genotype-1-infected subjects (Abstract 13). Paper presented at: 47th Annual Meeting of the European Association for the Study of the Liver (EASL); 2012 April 18–22; Barcelona, Spain.
3. Gilead Sciences press release. Available at: http://investors.gilead.com/phoenix.zhtml?c=69964&p=irol-newsArticle&ID=1761988&highlight=. Accessed on November 28, 2012).
4. Hassanein T, Lawitz E, Crespo I, et al; the ATOMIC Investigators. Once-daily sofosbuvir (GS-7977) plus PEG/RBV in treatment-naive patients with HCV genotype 1, 4, and 6 infection: The ATOMIC study (Abstract 230). Paper presented at: 63rd Annual Meeting of the American Association for the Study of Liver Diseases (AASLD); 2012 November 9–13; Boston, MA.
5. Hézode C,Shiffman ML, Cooper C, et al. Ritonavir-boosted danoprevir plus PegIFN alfa-2a/ ribavirin (P/R) demonstrates up to 100% SVR24 with 12 or 24 weeks of total treatment in treatment-naive patients with HCV genotype 4 infection in the DAUPHINE study (Abstract 760). Paper presented at: Paper presented at: 63rd Annual Meeting of the American Association for the Study of Liver Diseases (AASLD); 2012 November 9–13; Boston, MA.
6. Rodriguez-Torres M, Ishak L, Fontana D, et al; EMERGE Study Group. Peginterferon lambda-1a (LAMBDA) shows superior viral response with improved safety and tolerability versus peginterferon alfa-2a (alfa-2a) in patients with chronic HCV infection (G 1/2/3/4): EMERGE phase 2b through week 12 results. Paper presented at: 22nd Conference of the Asian Pacific Association for the Study of the Liver (APASL); 2012. February 16—19; Taipei, Taiwan.
7. Muir A, Hilson J, Gray T, et al; EMERGE Study Group. Peginterferon lambda-1a (lamdba) compared with peginterferon alfa-2a (alfa) in treatment naive patients with HCV genotypes 1 or 4: SVR24 results from EMERGE phase 2b (Abstract 214). Paper presented at: 63rd Annual Meeting of the American Association for the Study of Liver Diseases (AASLD); 2012 November 9–13; Boston, MA
8. Sulkowski MS, Sherman KE, Soriano V, et al.; Study 110 Team. Telaprevir in combination with peginterferon alfa-2a/ribavirin in HIV/HCV co-infected patients: SVR24 final study results (Abstract 54). Paper presented at: 63rd Annual Meeting of the American Association for the Study of Liver Diseases (AASLD); 2012 November 9–13; Boston, MA.
9. Kirby B, Mathias A, Rossi S, et al. No clinically significant pharmacokinetic drug interactions between sofosbuvir (GS-7977) and HIV antiretrovirals atripla®, rilpivirine, darunavir/ritonavir, or raltegravir in healthy volunteers (Abstract 1877). Paper presented at: 63rd Annual Meeting of the American Association for the Study of Liver Diseases (AASLD); 2012 November 9–13; Boston, MA.
10. Hézode C, Dorival C, Zoulim F, et al. Safety and efficacy of telaprevir or boceprevir in combination with peginterferon alfa/ribavirin, in 455 cirrhotic non responders. Week 16 analysis of the French early access program (ANRS CO20-CUPIC) in real-life setting (Abstract 51). Paper presented at: 63rd Annual Meeting of the American Association for the Study of Liver Diseases (AASLD); 2012 November 9–13; Boston, MA.
11. Gallegos-Orozco JF, Chervenak AE, Carey EJ, et al. Liver transplant center focused experience with peginterferon alfa-2a, ribavirin and telaprevir therapy in patients with genotype 1 hepatitis C cirrhosis (Abstract 53). Paper presented at: 63rd Annual Meeting of the American Association for the Study of Liver Diseases (AASLD); 2012 November 9–13; Boston, MA.
12. Burton JR, O’Leary J, Verna EC, et al. A multicenter study of protease inhibitor-triple therapy in HCV-infected liver transplant recipients: report from the CRUSH-C group (Abstract 211). Paper presented at: 63rd Annual Meeting of the American Association for the Study of Liver Diseases (AASLD); 2012 November 9–13; Boston, MA.
13. Fontana RJ, Bifano M, Hindes R, et al. First ever successful use of daclatasvir and GS-7977, an interferon-free oral regimen, in a liver transplant recipient with severe recurrent hepatitis (Abstract 694). Paper presented at: 63rd Annual Meeting of the American Association for the Study of Liver Diseases (AASLD); 2012 November 9–13; Boston, MA.
14. Mathias A, Cornpropst M, Clemons D, et al. No clinically significant pharmacokinetic drug-drug interactions between sofosbuvir (GS-7977) and the immunosuppressants, cyclosporine A or tacrolimus in healthy volunteers (Abstract 1869). Paper presented at: 63rd Annual Meeting of the American Association for the Study of Liver Diseases (AASLD); 2012 November 9–13; Boston, MA.
15. Ouwerkerk-Mahadevan S, Simion A, Mortier S, et al. No clinically significant interaction between the investigational HCV protease inhibitor TMC435 and the immunosuppressives cyclosporine and tacrolimus (Abstract 80). Paper presented at: 63rd Annual Meeting of the American Association for the Study of Liver Diseases (AASLD); 2012 November 9–13; Boston, MA.
