December 2012 Update – Cure rates presented at the American Association for the Study of Liver Diseases (AASLD)
July 2012
By Tracy Swan and Karyn Kaplan
Dedicated to Michael Carden
A fabulous, kind, brilliant, and hilarious friend and colleague
1971–2012
Special thanks to Jules Levin
Introduction
Hepatitis C virus (HCV) infection is curable, although it still kills more than 365,000 people each year.[1] Successful treatment reduces the risk of liver-related illness and death, even in people who have cirrhosis,[2-5] but pegylated interferon—the backbone of HCV treatment—is also the major obstacle to treatment access, delivery, uptake, and completion.
Fortunately, a revolution in HCV drug development is under way: proof of concept for safe and effective all-oral, interferon-free regimens has been established, and dozens of drugs from different classes are in development.
Treatment advances are long overdue for an estimated 160 million people with chronic hepatitis C.[6] Although HCV progresses slowly, liver damage develops exponentially once serious liver scarring—called bridging fibrosis—occurs; almost 10% of this group will progress to cirrhosis each year.[7] People with cirrhosis are vulnerable to liver failure and hepatocellular carcinoma (HCC; liver cancer). By 2007, more people in the United States were dying from HCV complications than from HIV/AIDS.[8]
In mid-2011, approval of the first hepatitis C protease inhibitors—Merck’s boceprevir (Victrelis) and Vertex’s telaprevir (Incivek/Incivo)—marked the beginning of the direct-acting antiviral (DAA) era. Although both drugs must be used with pegylated interferon (PEG-IFN) and ribavirin (RBV), their use increases the likelihood of being cured, and offers the possibility of shortened treatment.[9-13]
In real life, enduring and administering treatment with an HCV protease inhibitor–based regimen has turned out to be more difficult than patients and clinicians were led to expect based on data from clinical trials. Treatment-experienced people with advanced liver disease face harsh—even life-threatening—side effects. They require vigilant monitoring by experienced physicians. Serious adverse events have been reported in 30% to 51% of people with cirrhosis, versus 9% to 14% among participants in phase III clinical trials.[14] The shortage of qualified and willing treaters—who must follow complex drug- and patient-specific treatment algorithms—combined with rumors of unfamiliar and worse-than-expected side effects, high prices, and anticipation of better drugs, have circumscribed HCV treatment uptake in the United States and Europe.
The longing for simple, easy-to-tolerate, interferon-free regimens is fed by industry hype. The current treatment paradigm—which involves consideration of host and viral genotype, HCV subtype, liver histology, treatment history, and response to treatment at designated time points—will become less complex in the coming years, hopefully evolving into DAA regimens that will cure everyone.
Who’s Special?
With all of this exciting information, there is nothing exciting for them.
—Gloria Searson, MSW
Founding Director, Coalition for Positive Empowerment (COPE)
People with poor prognostic factors and greater need for treatment are often lumped together as “special populations,” which makes it easier to exclude them from registration trials. Instead, pharmaceutical companies design trials for people who are easier to treat but do not reflect the demographics of the HCV epidemic. When drugs are approved, information on their safety and efficacy in African Americans, Latinos and Latinas, people with common comorbidities such as HIV and bleeding disorders, people over 65 years of age, and people with cirrhosis is often limited. Underrepresentation or outright exclusion of current and former drug users and people on medication-assisted treatment with methadone or buprenorphine is a chronic problem—which persists despite ample evidence that they can be successfully treated.[15–20]
Now that it is possible to treat HCV without interferon, it is inexcusable to delay clinical trials in people with urgent need for them. Nonetheless, people with decompensated cirrhosis as well as transplant candidates and recipients are excluded from pre-approval trials despite pressure from activists, regulators, and desperate patients and their physicians.
Resistance
There is no consensus on whether there are clinical consequences associated with HCV drug resistance. Some experts are convinced that it will limit future treatment options, while others tend to dismiss it, citing both the development of many new and potent DAAs from different classes, and studies documenting reversion to wild-type virus over time.
It has become clear that HCV treatment is more likely to fail when preexisting resistance is compounded by poor interferon sensitivity and lower drug concentrations.[21,22] In contrast, pretreatment drug resistance does not always preclude successful treatment in people who are sensitive to interferon.[21–23] More data are needed, but in the meantime, pretreatment IL28B testing and HCV subtyping may help to identify people who are vulnerable to resistance-associated treatment failure.
Drug-Drug Interactions
Drug-drug interactions can lower DAA concentrations to subtherapeutic levels, leading to drug resistance and treatment failure, or increase drug concentrations, leading to worsened side effects and drug toxicity.
Treating HCV in HIV/HCV-coinfected people is complicated by drug-drug interactions with antiretroviral agents and other medications, especially in people over 50 years of age, since polypharmacy (use of multiple medications) is more common in older HIV-positive people (see Table 4. Drug-Drug Interactions between HCV DAAs and HIV Antiretroviral Agents).[24,25]
Since injection drug use with unsterilized equipment is a major mode of transmission for HCV, many people with hepatitis C are on substitution therapy with methadone or buprenorphine. Transplant recipients need immunosuppressive therapy. Type 2 diabetes and psychiatric disorders are common among people with hepatitis C. Drug-drug interaction studies should be performed to determine whether methadone, buprenorphine, immunosuppressants, insulin-sensitizing agents, statins (to lower cholesterol), psychotropic medications, and antiretroviral agents can safely be used during HCV treatment. Serious clinical consequences of uncharacterized drug-drug interactions include overdose, graft rejection, muscle weakness, and rhabdomyolysis (muscle damage that can lead to kidney failure).[26]
Up-to-date and comprehensive information on DAA drug interactions is available from the University of Liverpool at http://www.hep-druginteractions.org/.
DAAs by Class
Nucleosides and Nucleotide Polymerase Inhibitors
Nucleosides and nucleotide polymerase inhibitors are a therapeutic backbone for interferon-free regimens.
PSI-7977, the nucleotide furthest along in development, has been hailed as a wonder drug for its potency, high resistance barrier, and activity across HCV genotypes, favorable side-effect profile, and once-daily dosing. Results from small phase II trials supported the notion that PSI-7977 could cure everyone, possibly without pegylated interferon, and perhaps even as a monotherapy. On November 21, 2011, Gilead announced its plan to purchase Pharmasset for US$11 billion dollars; the sale went through in early 2012. Thus, PSI-7977 became GS-7977.
Since then, it has become clear that the wonder drug may need some help if it is indeed to be a cure-all. High relapse rates in people treated with monotherapy, prior null responders with HCV genotype 1, and treatment-experienced people with HCV genotypes 2 and 3 indicate the need for longer therapy and, possibly, other DAAs.
NS5a Inhibitors
NS5a inhibitors are active against all HCV genotypes, and they are potent, despite a low barrier to drug resistance. This class of drugs is moving into a co-anchor role in interferon-free regimens based on daclatasvir’s large safety database, favorable side-effect profile, once-daily dosing, and performance with other DAAs (GS-7977 and asunaprevir). Many drugs in this class feature once-daily dosing and pan-genotypic activity. Next-generation NS5a inhibitors are likely to have a higher resistance barrier.
HCV Protease Inhibitors
HCV protease inhibitors were the first class of DAAs to be approved. Subsequent versions will be optimized. The second batch of protease inhibitors is now in phase III; they will offer once-daily (versus thrice-daily) dosing, simpler treatment algorithms, and the prospect of greater efficacy. Tolerability may be better than that of first-generation protease inhibitors, despite side effects such as photosensitivity, abnormal elevations in bilirubin (a yellowish fluid created when the liver breaks down red blood cells), nausea, and vomiting. The next generation of protease inhibitors may be active against multiple genotypes and drug-resistant virus.
Non-Nucleoside Polymerase Inhibitors
Enthusiasm for this class of drugs has increased in the wake of its contribution to interferon-free regimens. Non-nucleosides generally have a low resistance barrier and are active only against genotype 1, but it may be possible to combine drugs from this class, as they target different sites (thumb- versus palm region of the HCV genetic structure).
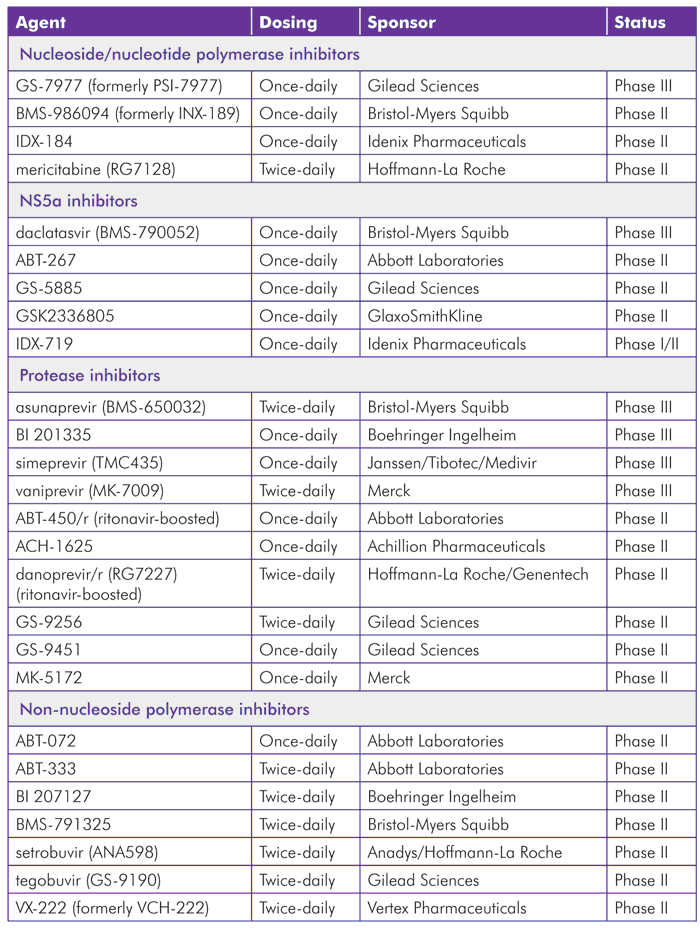
TABLE 1. Direct-Acting Antivirals in Phase II and Phase III
(For more detailed listing of current trials for each drug by class, see Tables 6 to 9)
The Next Generation
Fierce competition for the most effective and most tolerable regimen—with the shortest treatment duration—continues, with the focus on HCV genotype 1. The next drugs likely to be approved to treat hepatitis C are once-daily protease inhibitors (TMC435 and BI 201335). Both are being developed with pegylated interferon and ribavirin (PEG-IFN/RBV), and with other DAAs in interferon-free trials.
Drugs from other classes are also moving closer to the clinic: daclatasvir, an NS5a inhibitor, and GS-7977, a nucleotide polymerase inhibitor, are in phase III. Data from these trials are important for informing and optimizing DAA use in interferon-free regimens.
Simeprevir (TMC435)
Janssen’s once-daily HCV protease inhibitor, simeprevir, is currently in phase III trials.
PILLAR, a phase IIb trial in 368 treatment-naive people with HCV genotype 1, compared simeprevir dose (75 mg vs. 150 mg) and duration (12 vs. 24 weeks), in combination with PEG-IFN/RBV. Early responders were eligible for shortened treatment; otherwise, they continued on PEG-IFN/RBV for 48 weeks. Overall, the highest SVR rates (sustained virological response; HCV RNA becomes undetectable and remains undetectable for 24 weeks after treatment; regarded as a cure) and lowest relapse rates were seen with the 150 mg dose, especially with 24 weeks of triple therapy. In addition, SVR rates in the 150 mg dosing arms did not differ according to HCV subtype (genotype 1a or 1b), supporting use of the 150 mg dose in phase III studies.
Most participants (79–86%) were eligible for shortened treatment, forgoing the extra 24 weeks of PEG-IFN/RBV; and almost all early responders (93–96%) achieved SVR. As expected, the highest SVR rates were seen in people with the IL28B CC genotype (which is associated with interferon sensitivity and greater likelihood of SVR), whether or not they received simeprevir. In the IL28B CT and TT (harder-to-treat) groups, SVR was highest (78%) in the 150 mg dosing group, and lowest in the placebo group (50%). Oddly, SVR in the arm treated with PEG-IFN/RBV and placebo was 65%, higher than what has usually been reported.
Grade 3 or 4 adverse events occurred in 32% of the simeprevir arms, versus 35% of the placebo arm. Serious adverse events were twice as common in the placebo arm (13% vs. 6.5%), possibly due to shorter duration of PEG-IFN/RBV among most of the participants in the simeprevir groups. These events led to treatment discontinuation in 3.6% of the simeprevir groups, versus 5.2% in the placebo arm. The five most common adverse events among people treated with simeprevir were fatigue, flu-like symptoms, itching, headache, and nausea; the incidence of rash, anemia, and neutropenia (a decrease in white blood cells that fight off bacterial infections) did not differ significantly by treatment group. Transient elevations in bilirubin levels were seen in people who were treated with 150 mg of simeprevir; these elevations were attributed to blocked drug transporters (proteins that move drugs in and out of cells).[27]
ASPIRE, a phase IIb trial in prior relapsers, partial responders, and null responders with HCV genotype 1, looked at different doses (100 mg vs. 150 mg) and duration (12 vs. 24 vs. 48 weeks) of treatment with simeprevir with PEG-IFN/RBV. The best results were seen in the 150 mg dosing groups: 85% of prior relapsers, 75% of prior partial responders, and 51% of null responders achieved SVR. Although HCV subtype did not make a difference among relapsers, prior partial and null responders with HCV genotype 1a were less likely to be cured, as were people with more advanced liver damage; of note, 31% of prior null responders with cirrhosis were cured.[28]
SVR rates were similar in study participants (regardless of preexisting resistance) who received the 150 mg dose (vs. 100 mg). On-treatment viral breakthrough and posttreatment relapse rates were lower with the 150 mg dose (9% vs. 13% for breakthrough; 9% vs. 11% for relapse). After treatment, drug resistance was seen in 42 of 43 people who experienced breakthrough, and in 34 of 36 people who relapsed. People with HCV genotype 1a were more likely to have the R155K mutation by itself or with additional mutations, whereas people with HCV genotype 1b had the D168V mutation.[29]
Participants in the 150 mg arm had more grade 3 and 4 adverse events than those in the 100 mg or placebo arms (36% vs. 28% vs. 26%, respectively), and discontinuation rates were 9% (150 mg) versus 7% (100 mg) versus 5% (placebo). The most frequent adverse events—experienced by more than 25% of study participants—were headache, fatigue, flu-like symptoms, and itching. Other side effects included rash in 30%, 23%, and 18%, respectively; of note, severe rash was reported in 0.5% of participants treated with simeprevir, and photosensitivity in 2–6% of the simeprevir groups versus 2% of the placebo group. Laboratory abnormalities were similar across treatment groups, with the exception of mild, reversible elevations in bilirubin in simeprevir recipients.[28]
BI 201335
Boehringer Ingelheim’s once-daily HCV protease inhibitor, BI 201335, has entered phase III.
SILEN-C1 was a four-arm phase II trial in 429 treatment-naive people with HCV genotype 1. Participants were assigned to treatment with 120 mg or 240 mg of BI 201335 (or placebo) once daily, plus PEG-IFN/RBV. The entire 120 mg dosing arm and half of the 240 mg dosing arm began treatment with a three-day PEG-IFN/RBV lead-in. In the 240 mg dosing arm, early responders were randomized to either stop treatment at 24 weeks or continue with a 24-week PEG-IFN/RBV “tail”; the remaining study participants took 120 mg of BI 201335 or placebo for 24 weeks, plus 48 weeks of PEG-IFN/RBV.
In the placebo arm, 56% achieved SVR, while SVR in the BI 201335 groups ranged from 71% to 83%. The highest rate of SVR occurred in the no-lead-in 240 mg arm. In the response-guided 240 mg arm, 87% of participants required only 24 weeks of treatment.
In people with the IL28B CC genotype, 100% of the 240 mg group and 82% of the placebo group achieved SVR. High-dose BI 201335 significantly increased cure rates in people with non-CC genotypes to 71%, versus 41% in the placebo arm. SVR did not differ significantly by HCV subtype (82% in genotype 1a vs. 84% in genotype 1b).
Severe adverse events were reported in 11.8% of those in the 120 mg BI 201335 arm, 12.8% to 15.9% in the 240 mg arms, and 4.2% in the placebo arm. These led to discontinuations in 4.4% of those in the 120 mg dosing arm, 5.4% to 11.6% in the 240 mg dosing arms, and 1.4% in the placebo arm. One death was reported in the placebo arm (the cause was not described). Discontinuations for rash, jaundice, and photosensitivity occurred only in the 240 mg dosing arms.[30]
In SILEN-C2, 288 partial- or null responders with HCV genotype 1 were randomized into three treatment arms: 240 mg of BI 201335 once daily, with or without a three-day PEG-IFN/RBV lead-in, or BI 201335 twice daily with a three-day PEG-IFN/RBV lead-in. After 24 weeks of triple therapy, early responders assigned in the once-daily lead-in group either stopped all treatment or continued with an additional 24 weeks of PEG-IFN/RBV. Everyone else stopped BI 201335 at week 24 and continued PEG-IFN/RBV until week 48.
SVR rates ranged from 27% (once-daily, with lead-in) to 31% (twice-daily, with lead-in) up to 41% (once-daily, no lead-in group). The highest SVR in partial responders was 50%; among null responders it was 35% (both in the once-daily, no lead-in group). Among early responders in the once-daily lead-in group, those treated for 48 weeks were significantly less likely to relapse (21% vs. 60%, respectively). Most treatment failures were due to breakthrough during triple therapy and relapse.
Adverse events that were >10% more frequent during treatment with BI 201335 included rash, jaundice (from BI 201335–associated bilirubin elevations), nausea, diarrhea, and vomiting. These usually occurred less often in the once-daily dosing arm. Severe adverse events were reported in 14% of the once-daily dosing arms, and 27% in the twice-daily dosing arm, leading to treatment discontinuation in 4% to 6% (once-daily) versus 23.2% (twice-daily). Rash accounted for 1.3% (once-daily) versus 14.5% (twice-daily) of treatment discontinuation; 1.4% of the twice-daily group discontinued due to photosensitivity. Jaundice led to discontinuation in <1% of the once-daily group versus 1.4% in the twice-daily group.[31]
Results from SILEN-C3 further streamlined duration of therapy for treatment-naive people with HCV genotype 1. Treatment with 12 or 24 weeks of once-daily 120 mg BI 201335 and PEG-IFN/RBV (followed by response-guided PEG-IFN/RBV for 24 weeks) was equally effective, with SVR of 65% versus 73%.[32]
Asunaprevir (BMS-650032)
Asunaprevir, a twice-daily protease inhibitor from BMS is being studied with the company’s other DAAs (the NS5a inhibitor daclatasvir and BMS-791325, a non-nucleoside polymerase inhibitor), with or without pegylated interferon alfa or pegylated interferon lambda (see Meek as a Lambda) and ribavirin.Asunaprevir is active against HCV genotype 4. At higher doses, asunaprevir caused liver-enzyme elevations; the dose has been lowered from 600 mg twice daily to 200 mg twice daily.[21,33]
Asunaprevir’s twice-daily dosing may limit its use. A recently announced agreement between BMS and Janssen to study daclatasvir with Janssen’s protease inhibitor, simeprevir, may not bode well for asunaprevir.
GS-7977
Adding GS-7977 to PEG-IFN/RBV can shorten treatment and boost cure rates in non-cirrhotic treatment-naive people with HCV genotype 1, according to results from ATOMIC, a 332-person trial. ATOMIC compared 12 weeks of triple therapy to 24 weeks of triple therapy; a third arm looked at 12 weeks of triple therapy followed by GS-7977 plus ribavirin or GS-7977 monotherapy. Most of ATOMIC’s participants had an IL28B CT or TT genotype, and HCV genotype 1a.
In all treatment arms, HCV RNA rapidly became undetectable and remained undetectable throughout treatment. At 12 weeks after treatment completion, 90% of the 12-week treatment group had undetectable HCV RNA. In people treated for 24 weeks, HCV RNA remained undetectable 4 weeks after treatment completion in 92%. No viral breakthrough occurred; there were four relapses. To date, no evidence of S282T, a mutation associated with nucleotide resistance, has been detected among relapsers; results from resistance testing using deep sequencing are pending.
Serious adverse events were reported in 10% (5/52) of the people in the 12-week treatment arm, and 8% (4/52) discontinued treatment; one discontinuation was attributed to GS-7977. In the 24-week treatment groups, 5% (4/125) had a serious adverse event, and 15% (12/125) discontinued treatment due to adverse events. Of these adverse events, 5% (6/125) were related to GS-7977. In the groups treated with 12 weeks of triple therapy followed by 12 weeks of GS-7977 plus ribavirin or GS-7977 monotherapy, 4% (3/156) experienced a serious adverse event, 4% (6/156) discontinued treatment, and <1% (1/156) had adverse events related to GS-7977.
The most common adverse events, reported in >15% of study participants, were fatigue, headache, nausea, insomnia, chills, rash, anemia, fever, appetite loss, diarrhea, and neutropenia. Laboratory abnormalities improved quickly after discontinuation of pegylated interferon.[34]
Daclatasvir (BMS-790052)
Bristol-Myers Squibb’s first-in-class, once-daily, pan-genotypic NS5a inhibitor, daclatasvir, is in phase III. Daclatasvir is likely to be a therapeutic backbone, since it has been studied—and is effective—in interferon-free regimens with GS-7977 or asunaprevir (BMS-650032). Daclatasvir is also being studied with BMS-986094 (formerly INX-189), in triple therapy (with either pegylated interferon alfa or pegylated interferon lambda, plus ribavirin), and in quadruple therapy (with asunaprevir, pegylated interferon, and ribavirin).
Danoprevir/r
Hoffmann-La Roche and Genentech’s danoprevir/r (RG7227) is a twice-daily, ritonavir-boosted HCV protease inhibitor with activity against HCV genotypes 1, 4 and 6. DAUPHINE, an ongoing phase II trial in 421 treatment-naive people with HCV genotypes 1 and 4, is comparing doses (200, 100, and 50 mg danoprevir, boosted with 100 mg ritonavir, twice-daily) and response-guided therapy with danoprevir/r plus PEG-IFN/RBV. At 12 weeks after treatment completion, HCV RNA was undetectable in 86% of the highest-dosing arm, 77% of the 100 mg arm, and 65% of the 50 mg arm.
Response to treatment in the 200 mg dosing arm did not differ according to HCV subtype or IL28B genotype; at 12 weeks after treatment completion, 88% of people with HCV subtype 1a and an IL28B non-CC genotype had undetectable HCV RNA. Across all dosing arms, HCV RNA remained undetectable 12 weeks after treatment completion in 100% of people with HCV genotype 4.
In the response-guided therapy arm, 76% of early responders (who were treated for 12 weeks) and 67% of late responders (treated for 24 weeks) maintained undetectable HCV RNA 12 weeks after treatment completion, bringing the overall total to 72%.
One death occurred during the trial—from sudden heart attack, in a participant with preexisting diabetes and hypertension—it was considered unrelated to study drugs. Adverse events were reported in virtually all study participants. Side effects from ritonavir, which is used to boost danoprevir levels, increased the likelihood of more than one serious adverse event among people in the danoprevir/r arms (range 4–9% vs. 1% for placebo). The rate of danoprevir/r-related treatment discontinuations was similar to the rate of PEG-IFN/RBV-associated discontinuations (3–7%, and 3–8%, respectively).
Common side effects (experienced by more than 15% of study participants) included fatigue, fever, chills, weakness, nausea, diarrhea, itching, rash, hair loss, headache, aching muscles and joints, insomnia, cough, and appetite loss. Diarrhea was the only side effect associated with danoprevir/r. Adding danoprevir/r did not increase rates of rash or anemia (known side effects of other HCV protease inhibitors). Most grade 3 and grade 4 lab abnormalities were neutropenia, reported in 22% to 38% of study participants.[35]
Meek as a Lambda?
A new, type III interferon, peginterferon lambda, may replace pegylated interferon alfa. Lambda interferon may have fewer side effects than alfa interferon, because there are fewer receptors for it outside of the liver.
EMERGE, an ongoing phase IIb trial in 526 treatment-naive, non-cirrhotic people with HCV genotypes 1, 2, 3, and 4, is comparing safety, tolerability, and efficacy of peginterferon lambda versus pegylated interferon alfa (in combination with ribavirin) for 24 to 48 weeks, depending on HCV genotype. Participants with genotypes 1 and 4 are still being followed in the study, but final results from 41 participants with genotype 2 and 3 are available. Participants were assigned to once-weekly injections of 120 μg, 180 μg, or 240 μg of peginterferon lambda, or 180 μg of pegylated interferon alfa plus daily ribavirin (the 180 microgram dose will be studied in phase III trials).
Cure rates were similar among people with HCV genotype 2 (70% of the 180 μg peginterferon lambda dosing arm vs. 66% for pegylated interferon alfa); in HCV genotype 3, 83% versus 40% were cured by lambda and alfa, respectively, although the number of participants (29 or 30 per study arm) makes it difficult to draw conclusions about efficacy.
There was no significant difference in serious adverse events between peginterferon lambda and pegylated interferon alfa, but fever, chills, and muscle and joint pain occurred less frequently with peginterferon lambda versus pegylated interferon alfa. Although the number of people in each dosing arm was small, there were marked differences in the rate of severe laboratory abnormalities. With peginterferon lambda, the incidence of neutropenia was 0% (vs. 27% with pegylated interferon alfa); anemia occurred in 7% (vs. 45%); and thrombocytopenia incidence was 0% (vs. 24%).
Unfortunately, the dreaded psychiatric side effects of interferon—depression, irritability, and insomnia—were more common with peginterferon lambda than with pegylated interferon alfa (≥40% vs. 33%), regardless of the dose.[36]
HCV Quadruple Therapy (“Quad”)
As HCV drug development advances, optimizing treatment for people who are unlikely to respond—even if it means keeping interferon on board—should be prioritized (particularly since DAA regimens seem to cure people who are the easiest to treat successfully).
Experts have long recognized the immune system’s critical role in successful HCV treatment. It is paradoxical that some people with poor interferon sensitivity (demonstrated by null response) are more likely to be cured by quad therapy (two DAAs from different classes plus PEG-IFN/RBV) than by interferon-free regimens. Yet quad therapy has demonstrated efficacy in null responders as well as in treatment-naive people.[23,33,37,38]
So far, people with HCV genotype 1a, especially null responders to interferon-based treatment, seem to have the most to gain from quad. Over 90% of null responders in a clinical trial—most with HCV genotype 1a, and all with IL28B CT or TT genotypes—maintained undetectable HCV RNA throughout treatment with quad, and for four weeks afterward.[23] Identifying those most likely to benefit from quad is challenging in the era of all-DAA trials, and likely to make enrollment and retention difficult unless pegylated interferon is used only as rescue therapy.
Welcome to an Interferon-Free World
The year 2012 has ushered in the era of interferon-free therapy. Most trials have been in people who are treatment-naive, without cirrhosis. It is time for DAA trials to move into populations with the greatest need, informed by earlier trials in easier-to-treat populations.
Some of the factors associated with response to interferon-based treatment still apply when interferon is removed, and others have been identified. Determinants of successful treatment with DAAs include:
- Treatment-naive versus treatment-experienced;
- HCV subtype 1b versus 1a;
- IL28B genotype CC versus CT or TT genotype (although this can sometimes be overcome with a higher dose);
- Pretreatment IP-10 (interferon gamma–inducible protein 10) level: low versus high;
- Drug potency and resistance barrier;
- Drug concentration;
- Liver histology: mild-to-moderate liver damage versus cirrhosis;
- Baseline resistance;
- Appropriate treatment duration, according to regimen and population;
- Aggressive side effects management;
- Adherence to treatment; and
- Drug tolerability and safety: DAAs are described in press releases and at conferences as being “generally well tolerated,” despite a range of adverse events and laboratory abnormalities that can be debilitating, or even life-threatening.
Getting to the Best: Clinical Collaborations
Without a public-private research partnership, opportunities for best-in-class treatment regimens are lost.
DAAs can be combined into regimens when drugs targeting different steps in the HCV life cycle are at a similar stage of development. Sometimes, a company is not able to combine its own drugs into regimens. Clinical collaboration among companies—an approach used in HIV—facilitates development of DAA regimens, benefiting study volunteers and, ultimately, patients as well as the companies who sell these drugs.
Pharmasset and Bristol-Myers Squibb (BMS) launched a clinical collaboration in January 2011, combining PSI-7977 (a nucleotide polymerase inhibitor) and the NS5a inhibitor daclatasvir into a once-daily, interferon-free regimen active against genotypes 1, 2, and 3. In July 2011, Pharmasset entered a clinical collaboration with Tibotec (now Janssen), creating another once-daily, interferon-free regimen with PSI-7977 and simeprevir (TMC435; an HCV protease inhibitor). In December 2011, BMS and Janssen announced plans to launch a phase II trial in mid-2012 combining daclatasvir with simeprevir. In April, the companies declared their intention to continue collaboration by advancing the combination into phase III, and to study drug-drug interactions between simeprevir and BMS-986094 (formerly known as INX-189; a nucleotide polymerase inhibitor). These collaborations are examples of best practices in drug development.
In 2012, Gilead Sciences purchased Pharmasset for US$11 billion dollars. Although Gilead is supporting ongoing clinical trials with BMS and Janssen, its future participation in clinical collaborations is uncertain. Gilead has six other DAAs from four different classes in clinical development: two non-nucleoside polymerase inhibitors, two protease inhibitors, a nucleoside polymerase inhibitor, and an NS5a inhibitor (which it quickly advanced into a trial with GS-7977).
Gilead may decide not to collaborate with anyone—regardless of the benefit to patients—in order to gain control of the market. Failure to collaborate will force patients to wait for an in-house combination, provided all goes well with the development of BMS’s nucleotide and Gilead’s NS5a inhibitor. Unfortunately, both of these drugs are in an earlier stage of development than are daclatasvir and GS-7977.
Waiting for the Cure
Reporting posttreatment results as early as possible has become customary at scientific meetings. But the wait to determine a cure may take longer than 24 weeks. Without interferon, SVR-24 is called into question.
When Is a Cure Really a Cure?
Early response rates may ultimately become predictive of cure rates, but more data are needed to confirm their validity. At present, their predictive value is unclear, since relapse occurs at different time points, depending on drug, regimen, and patient-specific characteristics. For example, in ELECTRON, after 12 weeks of GS-7977 and ribavirin, 9 of 10 null responders with HCV genotype 1 relapsed within 4 weeks; a later relapse, at 8 weeks posttreatment, was reported in 1 of 15 people in another arm of ELECTRON (treatment-experienced people with HCV genotypes 2 and 3).[39,40] A single late relapse—at posttreatment week 36—occurred in a treatment-naive study participant treated with ribavirin plus two experimental drugs from Abbott Laboratories, a boosted protease inhibitor (ABT-450/r) and a non-nucleoside polymerase inhibitor (ABT-072).[41]
SVR-4 (sustained virological response at 4 weeks after treatment completion): HCV RNA becomes undetectable during treatment and remains undetectable 4 weeks afterward. SVR-4 is not validated as a predictor of treatment outcome, since relapse may take longer than 4 weeks to occur.
SVR-12 (sustained virological response at 12 weeks after treatment completion): HCV RNA becomes undetectable during treatment and remains undetectable 12 weeks afterward. The U.S. Food and Drug Administration (FDA) accepted SVR-12 as an endpoint. SVR-12 is tightly correlated with SVR-24 in interferon-based regimens, since relapse almost always occurs within 12 weeks of treatment completion.
SVR-24 (sustained virological response at 24 weeks after treatment completion): HCV RNA becomes undetectable during treatment and remains undetectable for 24 weeks afterward. SVR-24 after interferon-based treatment is considered to be a cure, is durable (the late relapse rate is <1%), and is known to decrease the incidence of liver-related illness and death.[42-45]
DAA Combinations: In the Treatment-Naive
Daclatasvir (BMS-790052) and GS-7977, with and without Ribavirin (HCV Genotypes 1, 2, and 3)
The exciting—but possibly short-lived—clinical collaboration between BMS and Gilead identified a winning combination of once-daily DAAs for 88 non-cirrhotic people with HCV genotypes 1, 2, and 3. A month after completing 24 weeks of treatment with GS-7977 and daclatasvir (with or without ribavirin), the SVR-4 rate was 100% in people with HCV genotype 1, and >85% in those with HCV genotypes 2 and 3, regardless of ribavirin use.[46] The trial is now looking at 12 weeks of treatment with the same drugs.
GS-7977 and Ribavirin (HCV Genotype 1)
In ELECTRON, 25 non-cirrhotic people with HCV genotype 1 were treated with GS-7977 and ribavirin for 12 weeks. Four weeks after treatment completion, HCV RNA remained undetectable in 22 of 25 study participants, yielding an SVR-4 rate of 88%.[40] Additional trials of shorter regimens are under way.
There were no discontinuations; adverse events were mild to moderate: four people each experienced a single adverse event: headache, nerve pain, chest pain, and vomiting; one experienced a drop in white blood cells.[39]
However, the same regimen was less effective for people with genotype 1 in QUANTUM, which is comparing 12 versus 24 weeks of treatment. In the 12-week treatment group, only 10 of 17 people had undetectable HCV RNA four weeks after finishing treatment, translating to an SVR-4 rate of 59%. QUANTUM participants differed from ELECTRON’s; only 16% (3/19) had the IL28B CC genotype, versus 44% (11/25) of ELECTRON’s participants. In QUANTUM, all of the relapses occurred in people with the IL28B CT or TT genotype. QUANTUM included people with cirrhosis—who have lower exposure to GS-7977 than people without cirrhosis—whereas ELECTRON did not.[47,48]
GS-7977 plus Ribavirin (with or without Pegylated Interferon); GS-7977 Monotherapy (HCV Genotypes 2 and 3)
GS-7977 has been studied in non-cirrhotic people with HCV genotypes 2 and 3. In the ELECTRON trial, 10 people were treated for 8 weeks, and 40 people were treated for 12 weeks, with GS-7977 and ribavirin with 0, 4, 8, or 12 weeks of pegylated interferon; 100% of them achieved SVR-24. A 12-week GS-7977 monotherapy arm was added; 60%, or 6 of 10 people, achieved SVR-24.
Adverse events were fewer in the interferon-free arm; 40% of participants reported experiencing one adverse event (vs. 50–72% in the interferon arms)—these were headache, fatigue, and aching muscles. No grade 3 or grade 4 laboratory abnormalities occurred among participants in the interferon-free arm, whereas one case of grade 3 anemia, three cases of grade 3 lymphopenia, thirteen cases of grade 3 neutropenia, nine cases of leukopenia, and four cases of grade 4 neutropenia were reported among participants who received interferon.[39]
ABT-450/r and ABT-072 plus Ribavirin (HCV Genotype 1)
Abbott’s PILOT trial enrolled 11 non-cirrhotic people with HCV genotype 1 and an IL28B CC genotype, who were treated with 12 weeks of ABT-450/r (a ritonavir-boosted HCV protease inhibitor), ABT-072 (a non-nucleoside polymerase inhibitor), and ribavirin. One relapse occurred at 8 weeks after treatment completion. Although 91% achieved SVR-24, a late relapse at 36 weeks posttreatment lowered SVR to 82%. Both unsuccessfully treated people had HCV genotype 1a; although neither had evidence of resistance at baseline, protease resistance was found after the early relapse, and polymerase resistance after the late relapse.[41]
ABT-450/r and ABT-333 plus Ribavirin (HCV Genotype 1)
Abbott’s COPILOT trial included non-cirrhotic treatment-naive, interferon-ineligible, or treatment-experienced, interferon-intolerant participants with HCV genotype 1. The SVR-12 rate among COPILOT’s treatment-naive participants ranged from 93% to 95% after 12 weeks of triple therapy with ABT-450/r (a ritonavir-boosted protease inhibitor) once daily, ABT-333 (non-nucleoside polymerase inhibitor) twice daily, and ribavirin.
Serious adverse events included elevated bilirubin (managed with ribavirin dose reduction), fatigue, pain, and vomiting; none led to treatment discontinuation. One participant discontinued treatment after two weeks due to grade 3 liver-enzyme elevations, which resolved after treatment discontinuation.
COPILOT’s most common side effects, experienced by >20% of study participants, were: fatigue, nausea, headache, dizziness, insomnia, rash, itching, and vomiting. Laboratory abnormalities (which included six cases of elevated bilirubin, attributed to ABT-450/r, and two cases of elevated creatinine) resolved during treatment.[49]
BI 201335 and BI 207127 plus Ribavirin (HCV Genotype 1)
Boehringer Ingelheim’s five-arm SOUND-C2 trial identified a DAA regimen that is effective for treatment-naive people with HCV genotype 1b, and people with HCV genotype 1a who have the IL28B CC genotype. SOUND-C2, a 368-person trial, compared 16 to 40 weeks of treatment with BI 201335, a once-daily HCV protease inhibitor, and twice- versus thrice-daily BI 207127, a non-nucleoside polymerase inhibitor, with or without ribavirin. SVR-12 ranged from 39% in the no-ribavirin arm to 68% in people treated with 28 weeks of BI 201335 and twice-daily BI 207127 plus ribavirin.
Researchers found significant differences in SVR rates, according to HCV subtype (genotype 1a versus 1b) and IL28B genotype (CC versus non-CC). In the 68% SVR-12 treatment arm, SVR-12 was 43% in genotype 1a versus 83% for genotype 1b, and 64% for non-CC genotype versus 79% for CC genotype. Overall, SVR-12 among people with HCV genotype 1a, non-CC, was 32% versus 75% (1a, CC); in HCV genotype 1b, SVR-12 was 82% in non-CC, and 84% in CC.[50]
Tolerability of twice-daily BI 207127 was better than that of thrice-daily dosing, with no severe adverse events reported in the 28-week arm. Moderate adverse events led to nine discontinuations (jaundice [N = 2], vomiting [N = 3], and diarrhea [N = 4]) in the 28-week treatment arm. Laboratory abnormalities in the 28-week arm are as follows: elevated bilirubin (a previously reported side effect of BI 201335, attributed to blocked drug transporters): 26% grade 3, and 10% grade 4; ALT elevations: 3% grade 3; and anemia: 2% (one case each of grade 3 and grade 4).[50,51]
SOUND-C2 offers the first glimpse of DAA safety and efficacy in people with compensated cirrhosis. A group of 37 SOUND-C2 participants (or 10%) had cirrhosis; more than half (N = 25) had HCV genotype 1b. Overall, the SVR-12 with thrice-daily BI 207127 was 57%, versus 54% for twice-daily BI 207127 (and 33% for the no-ribavirin arm). As expected, SVR-12 was higher in HCV genotype 1b than HCV genotype 1a in all treatment arms; in the 28-week, twice-daily dosing arm, it was 71% (vs. 33%). Although viral breakthrough rates were higher in the twice-daily dosing arm than the thrice-daily dosing arm (38% vs. 19%), relapse rates were lower (0% vs. 8%).
In participants with cirrhosis, tolerability of twice-daily BI 207127 was superior to thrice-daily dosing. All participants in the twice-daily arm experienced adverse events; serious adverse events were reported in 15% (N = 2) of participants in the twice-daily group, with one case of anemia leading to treatment discontinuation. In the thrice-daily arm, 19% experienced serious adverse events that caused six people to discontinue treatment; these were rash, photosensitivity, and jaundice. Elevations in bilirubin—without liver dysfunction—were attributed to BI 201335.[52]
Additional trials are planned in people with HCV genotype 1b and HCV genotype 1a—CC only—due to high rates of viral breakthrough and relapse in people with HCV genotype 1a and non-CC genotypes.[50]
Danoprevir/r and Mericitabine, plus Ribavirin (HCV Genotypes 1 and 4)
Roche’s phase IIb study, INFORM-SVR, is combining response-guided therapy with danoprevir/r, a twice-daily ritonavir-boosted HCV protease inhibitor, and mericitabine, a twice-daily nucleoside polymerase inhibitor, with or without ribavirin for 12 to 24 weeks in non-cirrhotic people with HCV genotype 1. The original study design was modified after high relapse rates were observed in the 12-week treatment and ribavirin-free arms. Treatment was extended to 24 weeks, and ribavirin was given to all participants.
The majority of INFORM-SVR participants were male, had HCV genotype 1a, and non-CC genotypes. Of the 64 people treated for 24 weeks with all three drugs, 41% experienced SVR-12. People with HCV genotype 1b were more likely to achieve SVR-12 (71% versus 26% in HCV genotype 1a). In contrast, SVR-12 was more likely among people with non-CC genotypes (32% for CC versus 44% for non-CC), although only 4 people had HCV genotype 1b and CC genotype. Breakthrough rates were higher in people who did not receive ribavirin, and in HCV genotype 1a versus 1b. Resistance to danoprevir/r was observed in all patients who experienced viral breakthrough; mericitabine resistance was found in one person.
Almost all participants had more than one adverse event; a total of 567 mild-to-moderate events were reported among 83 people. The most common side effects, occurring in >10% of people were headache, fatigue, nausea, diarrhea, colds, insomnia, itching, weakness, dizziness, irritability, shortness of breath, cough, upset stomach, painful joints, and vomiting. As for laboratory abnormalities, one person experienced grade 3 anemia, four people had grade 3 lipid elevations, and one case each of grade 3 elevations in phosphate and lipase were observed.
A single serious adverse event, multiple myeloma, occurred 53 days after treatment completion and one person discontinued due to pain in the back of the throat (it was not specified whether or not this was a treatment-related adverse event).[53]
DAA Combinations in the Treatment-Experienced and Interferon-Ineligible/Intolerant
Results from interferon-free trials in treatment-experienced people and those ineligible for, or intolerant of, pegylated interferon have been mixed.
ABT-450/r and ABT-333 plus Ribavirin* (HCV Genotype 1)
Abbott’s COPILOT trial included 17 non-cirrhotic partial- or null responders with HCV genotype 1, who were treated for 12 weeks with ABT-450/r (a once-daily, ritonavir-boosted HCV protease inhibitor), ABT-333 (a twice-daily HCV non-nucleoside polymerase inhibitor), and ribavirin—the same regimen given to COPILOT’s treatment-naive participants.
In this treatment-experienced group, SVR-12 was 47% (vs. 93–95% among treatment-naive participants). During treatment, six viral breakthroughs—all but one in people with HCV genotype 1a—occurred in people with no pretreatment resistance; after breakthrough, resistance to both protease- and polymerase inhibitors was found in all of them. In the lone genotype 1b breakthrough, resistance to HCV protease inhibitors was found at baseline; resistance to both classes was detected after treatment. Relapse occurred in three participants, all with HCV genotype 1a; none had pretreatment resistance, but two of three had resistance to both drug classes after relapse.[49]
* COPILOT’s adverse events are described above, since it included treatment-naive participants.
Daclatasvir and Asunaprevir (HCV Genotype 1)
BMS has a highly effective in-house combination for non-cirrhotic null responders with HCV genotype 1b. After 24 weeks of treatment with dual DAAs (once-daily daclatasvir, and asunaprevir, a twice-daily protease inhibitor), SVR-24 was 77% among a group of 21 null responders and 23 interferon-ineligible/intolerant participants. This phase IIa, open-label trial was conducted in Japan, where HCV genotype 1b is highly prevalent. SVR-24 was higher among null responders (91%) than among interferon-ineligible/intolerant participants (64%).
Although 10 study participants had pretreatment resistance to daclatasvir, five of them achieved SVR-24. There were three viral breakthroughs, and four people relapsed; most had lower drug concentrations than people who achieved SVR-24. Researchers speculated that the combination of preexisting drug resistance and lower drug exposure could have led to treatment failure, since people with either one of these were successfully treated.
During treatment, five study participants experienced serious adverse events (high fever, gastroenteritis, and elevated bilirubin—which were unrelated to study drugs—and hypochondria). There were three discontinuations; two for liver-enzyme elevations and one for elevated bilirubin. The adverse events experienced by at least three participants included headache, cold, diarrhea, fever, stomach pain, malaise, constipation, back pain, and appetite loss. Grade 3 and 4 laboratory abnormalities included abnormal elevations in white blood cells, liver enzymes (alanine aminotransferase [ALT] and aspartate aminotransferase [AST]), bilirubin, and phosphorous.[21,22]
GS-7977 and Ribavirin (HCV Genotypes 1, 2, and 3)
GS-7977 is less effective for people who are treatment-experienced, regardless of HCV genotype. In the ELECTRON study, 9 of 10 non-cirrhotic null responders with HCV genotype 1 relapsed within four weeks of completing 12 weeks of treatment with GS-7977 and ribavirin. In this group, HCV RNA became undetectable within four weeks and remained undetectable throughout, suggesting that extending treatment and/or adding anther DAA may do the trick. The only cure occurred in a participant with characteristics associated with successful HCV treatment: she was a young Caucasian woman with the IL28B CC genotype and barely any fibrosis.
Among null responders, one person experienced anxiety, depression, and a sprained ankle; there was one case of anemia; and one case of low platelets (in a participant using warfarin, a blood thinner) was reported.[39]
The same regimen, 12 weeks of GS-7977 and ribavirin, has been studied in a group of treatment-experienced people with HCV genotypes 2 and 3 (defined as relapsers, partial responders, and null responders). There was SVR-4 in 80% (12 of 15) of the participants. An additional relapse was reported at 8 weeks after treatment completion. Adverse events were mild to moderate, with two cases of headache, and no laboratory abnormalities.[40]
DAAs in HIV/HCV-Coinfected People
Rapid HCV Progression vs. Slow Drug Development
Hepatitis C remains a common—and dangerous—coinfection among HIV-positive people. Worldwide, an estimated 5 million people are HIV/HCV-coinfected.[54] The benefits of antiretroviral therapy have been offset by an increased risk of death associated with hepatitis C among HIV/HCV-coinfected people.[55] In fact, end-stage liver disease secondary to hepatitis C is a leading cause of death among people with HIV/AIDS where antiretroviral therapy (ART) is widely available.[56-60]
Treating—and curing—hepatitis C in coinfected people significantly reduces the rate of progression to AIDS, death from AIDS and non-AIDS-related causes, as well as liver-related illness and death.[61,62] It is time to move interferon-free trials into HIV/HCV-coinfected people. As of mid-2012, there are no clinical trials of dual- or multiple DAAs or quad in HIV-positive people.
Trials of response-guided therapy and trials in coinfected people who are HCV treatment-experienced are planned or ongoing.
TABLE 2. HCV Treatment Trials for HIV/HCV-Coinfected People
Drug/Class/Sponsor |
Study Population |
Strategy |
Status |
|---|---|---|---|
|
boceprevir (protease inhibitor) National Institute of Allergy and Infectious Diseases |
HCV or HIV/HCV |
4-week PEG-IFN/RBV “lead-in” followed by response-guided triple therapy |
Phase IV; Open for enrollment |
|
BI 201335 (protease inhibitor) Boehringer Ingelheim |
HIV/HCV genotype 1, |
Response-guided triple therapy |
Phase III; Open for enrollment |
|
BMS-790052 (daclatasvir) (NS5a inhibitor) Bristol-Myers Squibb |
HIV/HCV genotype 1, treatment-naive |
24 weeks of triple therapy followed by 24-week |
Phase III; Open for enrollment |
|
boceprevir (protease inhibitor) National Institute of Allergy and Infectious Diseases |
HIV/HCV genotype 1, |
4-week PEG-IFN/RBV “lead-in” followed by response-guided triple therapy |
Phase III; Open for enrollment |
|
simeprevir (TMC435) (protease inhibitor) Janssen R&D Ireland |
HIV/HCV genotype 1, treatment-naive and treatment-experienced |
Response-guided triple therapy |
Phase III; Ongoing; no longer enrolling |
| Updated 10/2012
sofosbuvir (GS-7977, formerly PSI-7977) (nucleotide polymerase inhibitor) Gilead Sciences |
HIV/HCV genotypes 2 and 3, treatment-naive and treatment-experienced | GS-7977 plus weight based RBV for 12 or 24 weeks | Phase III;
N=115 Open for enrollment |
|
telaprevir (protease inhibitor) Janssen-Cilag International NV |
HIV/HCV genotype 1, with severe fibrosis or compensated cirrhosis who are ineligible for ongoing clinical studies of telaprevir |
12 weeks of triple therapy followed by a 36-week |
Phase III; Open for enrollment |
|
telaprevir (protease inhibitor) Vertex Pharmaceuticals |
HIV/HCV genotype 1, treatment-naive and treatment-experienced |
Response-guided triple therapy |
Phase III; Open for enrollment |
|
telaprevir (protease inhibitor) Tibotec |
HIV/HCV genotype 1, |
Response-guided triple therapy |
Phase IIIb; Not open as of 5/25/12 |
HCV Protease Inhibitors in HIV/HCV-Coinfected People
The first trials of HCV protease inhibitor–based therapy in HIV/HCV-coinfected people opened in 2009, prior to issuance of the FDA’s draft guidance for industryChronic Hepatitis C Virus Infection: Developing Direct-Acting Antiviral Agents for Treatment,which stipulated that single-arm prospective trials with historical controls could be used for HIV/HCV-coinfected patients “if trials in the HCV monoinfected population showed robust and substantial efficacy of the new DAA added to SOC [standard of care].”[63] Thus, both of these trials had a placebo arm, which may have slowed enrollment.
Adding boceprevir or telaprevir to PEG-IFN/RBV boosts cure rates among coinfected people. Two small, 48-week phase II trials found that both the safety profile and response to treatment with an HCV protease inhibitor plus PEG-IFN/RBV were similar, regardless of HIV status.[10,11,64,65]
The overall SVR-12 rate was 74% with telaprevir-based treatment (vs. 45% in the PEG-IFN/RBV control group). Participants began treatment with 12 weeks of triple therapy, followed by a 36-week “tail” of PEG-IFN/RBV.
Treatment was discontinued by 42% (16/38) of the participants in the telaprevir arm: 5% (2/38) for treatment failure; 8% (3/38) due to adverse events; and 32% (11/38) for various reasons, including noncompliance, lost to follow-up, and withdrawal of consent. In the control arm, 32% (7/22) stopped due to treatment failure, and 9% (2/22) discontinued due to relocation or because they did not want to remain in the trial.[64]
With boceprevir-based treatment, SVR-12 was 60% (versus 26% in the PEG-IFN/RBV control group). Participants began treatment with a four-week PEG-IFN/RBV lead-in, followed by 44 weeks of triple therapy.
Treatment was discontinued by 38% (24/64) of those in the boceprevir arm: 20% (13/64) due to adverse events; 9% (6/64) due to treatment failure; and the remainder (5/64) lost to follow-up, non-compliant, or did not want to continue participating in the trial. In the control arm, 53% (18/34) discontinued treatment: 9% (3/34) for adverse events, 41% (14/34) for treatment failure, and 3% (1/34) for reasons unrelated to treatment.
Unfortunately, boceprevir and telaprevir add side effects to a regimen that is already poorly tolerated.
TABLE 3.Adverse Events among HIV/HCV-Coinfected Study Participants
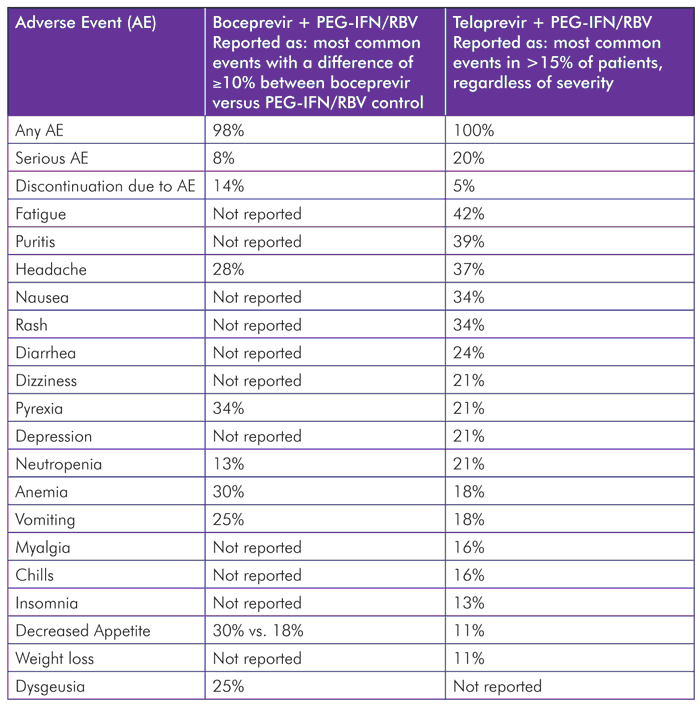
Sources:
Sherman KE, Rockstroh JK, Dieterich DT, et al. Telaprevir combination with peginterferon alfa-2a/ribavirin in HCV/HIV coinfected patients: 24-week treatment interim analysis (Abstract LB-8). Paper presented at: 62nd Annual Meeting of the American Association for the Study of Liver Disease; 2011 November 4–8; San Francisco, CA.
Sulkowski M, Pol S, Cooper C, et al. Boceprevir plus peginterferon/ribavirin for the treatment of HCV/HIV co-infected patients: interim on-treatment results (Abstract LB-37). Paper presented at: 49th Annual Meeting of the Infectious Diseases Society of America (IDSA 2011); 2011 October 20–23; Boston, MA.
TABLE 4. Drug-Drug Interactions between HCV DAAs and HIV Antiretroviral Agents
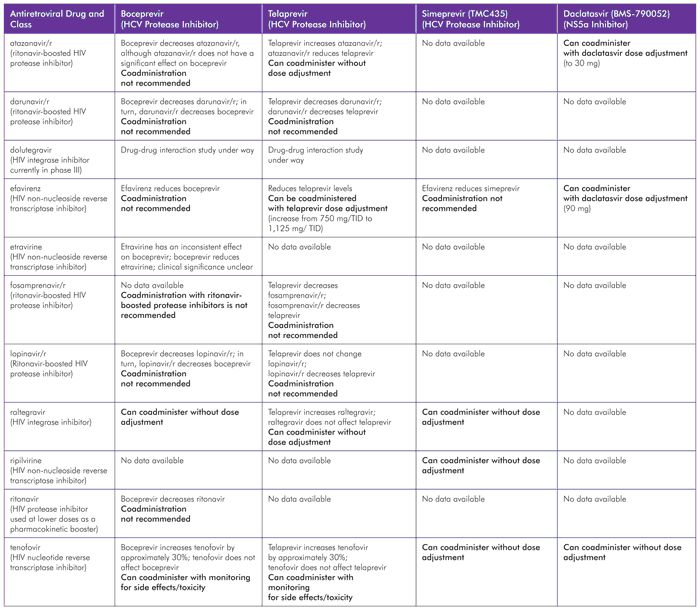
Sources:
Bifano M, Hwang C, OosterhuisB, et al. Assessment of HIV ARV drug interactions with the HCV NS5A replication complex inhibitor BMS-790052 demonstrates a pharmacokinetic profile which supports co-administration with tenofovir disoproxil fumarate, efavirenz, and atazanavir/ritonavir (Abstract 618). Paper presented at: 19th Conference on Retroviruses and Opportunistic Infections; 2012 March 5–8; Seattle, WA.
de Kanter C, Blonk M, Colbers A, et al. The influence of the HCV protease inhibitor boceprevir on the pharmacokinetics of the HIV integrase inhibitor raltegravir (Abstract 772 LB). Paper presented at: 19th Conference on Retroviruses and Opportunistic Infections; 2012 March 5–8; Seattle, WA.
Hammond K, Wolfe P, Burton J, et al. Pharmacokinetic interaction between boceprevir and etravirine in HIV/HCV seronegative volunteers (Abstract O_15). Paper presented at: 13th International Workshop on Clinical Pharmacology of HIV Therapy; 2012 April 16–18; Barcelona, Spain.
Hulskotte E, Feng H-P, Xuan F, et al. Pharmacokinetic interaction between the HCV protease inhibitor boceprevir and ritonavir-boosted HIV-1 protease inhibitors atazanavir, lopinavir, and darunavir (Abstract 771 LB). Paper presented at: 19th Conference on Retroviruses and Opportunistic Infections; 2012 March 5–8, 2012; Seattle, WA.
Kasserra C, Hughes E, Treitel M, et al. Clinical pharmacology of boceprevir: metabolism, excretion, and drug-drug interactions (Abstract 118). Paper presented at: 18th Conference on Retroviruses and Opportunistic Infections. 2011 February 27–March 2; Boston, MA.
Ouwerkerk-Mahadevan S, Sekar V, Peeters M, et al. The pharmacokinetic interaction of the HCV protease inhibitor TMC 435 with RPV, TDF, EFV or RAL in healthy volunteers (Abstract 49). Paper presented at: 19th Conference on Retroviruses and Opportunistic Infections; 2012 March 5–8; Seattle, WA.
Van Heeswijk R, Vandevoorde A, Boogaerts G, et al. Pharmacokinetic interactions between ARV agents and the investigational HCV protease inhibitor telaprevir in healthy volunteers (Abstract 119). Paper presented at: 18th Conference on Retroviruses and Opportunistic Infections; 2011 February 27–March 2; Boston, MA.
Up and Comers
Nucleoside and Nucleotide Polymerase Inhibitors
In January 2012, BMS purchased Inhibitex for approximately US$2.5 billion dollars, largely to get hold of INX-189 (now known as BMS-986094), a nucleotide in phase II. Data were expected in mid-May; hopefully, rumors about preclinical toxicity will not be substantiated.
Vertex has licensed two nucleotides from Alios Biopharma, ALS-2200 and ALS-2158, at a bargain price: US$60 million up front with a future payment of US$1.5 billion. They are currently in phase I, moving from healthy volunteers into a seven-day study in people with HCV. Data are expected in mid-2012.
Gilead’s GS-6620 is not expected to move out of phase I unless drug delivery can be optimized. Idenix has a preclinical nucleotide candidate, IDX19368.
NS5a Inhibitors
Achillion has two once-daily, pan-genotypic candidates in phase I. ACH-2928 has been studied in healthy volunteers and people with HCV. ACH-3102 may be active against resistant HCV; it is now being studied in healthy volunteers.
EDP-239, from Enanta and Novartis, is entering phase I; it is a once-daily drug and is active against multiple genotypes. Merck’s MK-8742 has entered a phase I trial in the Republic of Moldova; it may be active against resistant HCV and demonstrates activity against multiple genotypes, but may be less active against HCV genotype 2b. PPI-668 from Presidio Pharmaceuticals is a once-daily pan-genotypic NS5a inhibitor in phase Ia/Ib. Medivir has an unnumbered candidate in preclinical development.
Protease Inhibitors
Achillion’s pan-genotypic ACH-2684 is in phase I.
Non-Nucleoside Polymerase Inhibitors
Gilead’s GS-9669 is in phase I. Presidio’s PPI-338 is in preclinical development; it offers once-daily dosing and may be unlikely to interact with many commonly used drugs. TMC647, from Janssen and Medivir, is in preclinical development. In contrast, Pfizer’s filibuvir has not budged from a completed phase II trial launched in 2009.
Innovation without Access
Pharmaceutical companies and investment advisors have been avidly spinning results from small clinical trials of carefully selected participants into billions of dollars. Goldman Sachs predicted that the first DAAs would boost spending on HCV treatment in the next few years from US$3 billion to US$10 billion annually; other pundits forecast that the market for HCV drugs will swell to US$16 billion by 2015.
The hyperbole about new DAAs exists in sharp contrast to the lack of resources, infrastructure, and implementers needed to roll out programs to educate, test, diagnose, and treat millions of people. All over the world, people with HCV are hoping to gain access to a cure. Profit is leading a stampede over the basic human right to treatment for a potentially fatal—but curable—infection.
It does not matter how good the drugs are if people are undiagnosed, or cannot gain access to them. High drug prices will keep a cure out of reach for most of the 160 million people with hepatitis C.[6] Although drug pricing varies by country, the cost of HCV protease inhibitors is prohibitive in low- and middle-income countries, and limits access in wealthier countries. In the United States, where almost 50 million people currently have no medical coverage, the cost of an HCV protease inhibitor ranges from approximately US$32,000 to over US$52,000.[66]
Even without an HCV protease inhibitor, treatment with pegylated interferon and ribavirin is too expensive for people in most countries. Although ribavirin is available as a generic, and can be produced cheaply, Pegasys remains under patent by Hoffmann-La Roche in the United States, Europe, and Japan until 2017, and Merck’s PEG-Intron is under patent in the United States, Europe, and Japan until 2016, keeping prices high.
It is possible to provide pegylated interferon and ribavirin in resource-limited settings; a precedent has been set by activists and policy makers in Egypt and in Thailand, who have broadened access by negotiating lower prices for pegylated interferon (see Hepatitis C (HCV) Treatment Access: Spotlight on Thailand/Asia).
If the world is to benefit from therapeutic advances in HCV treatment, 2013 should become the year of implementation. It is time to prepare health care systems for the people who will enter them for testing, care, and treatment.
Nomenclature: The Terms, They Are a-Changing *
DAAs are a new therapeutic territory. Interferon-era terms, time frames, and endpoints may not be applicable to DAA trials. New terms to describe responses to interferon-free regimens will facilitate cross-drug and cross-regimen comparisons by providing consistency across studies. To this end, experts in the field have developed new nomenclature for DAA trials. They recommend specifying the assay’s lower limit of quantification, reporting viral decline in increments of 0.1 log10,and using these figures:
- W# to indicate week of treatment
- Q for quantifiable HCV RNA
- U for unquantifiable HCV RNA
- TD/TND to indicate whether or not target HCV RNA was detected
- LIW/D for duration of treatment lead-in, by weeks or days
TABLE 5. Nomenclature in Action
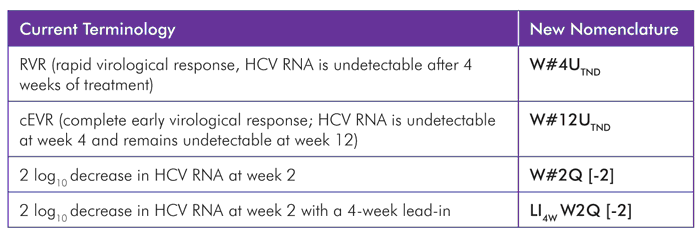
Source: Jensen DM, Wedemeyer H, Godofsky E, et al.; On Behalf of the Definitions/Nomenclature Working Group of the HCV Drug Development Advisory Group. Consensus recommendations for virologic nomenclature in DAA trials (Abstract 897). Paper presented at: 47th Annual Meeting of the European Association for the Study of the Liver; 2012 April 18–22; Barcelona, Spain.
*Data are reported in this chapter as they were presented, to avoid confusion.
RESOURCES
ClinicalTrials.gov (www.clinicaltrials.gov) provides information on HCV clinical trials and research.
HCV Advocate (www.hcvadvocate.org) offers conference reporting and an up-to-date HCV pipeline chart; available at: http://www.hcvadvocate.org/hepatitis/hepC/HCVDrugs.html.
HIV and Hepatitis.com (www.hivandhepatitis.com) offers news and conference reports.
NATAP (National AIDS Treatment Advocacy Project) (www.natap.org) offers comprehensive coverage of HCV and HIV/HCV coinfection.
TABLE 6. Nucleoside/Nucleotide Polymerase Inhibitors in Phase II and Phase III
Agent/Dose/Sponsor |
Phase/Population/Duration |
Comments |
|---|---|---|
|
Updated 10/2012 This drug is on clinical hold due to toxicity BMS-986094 25 mg, 50 mg, Once-daily Bristol-Myers Squibb |
Phase II Genotypes 2 and 3 |
All trials halted due to toxicity + RBV + PEG-IFN/RBV + daclatasvir (BMS-790052, an |
|
sofosbuvir (GS-7977, formerly PSI-7977)400 mg Once-daily Gilead Sciences |
Phase II and phase III Genotypes 2 and 3 Genotypes 1, 2, 3, 4, 5, & 6 |
Being studied as monotherapy + PEG-IFN/RBV + simeprevir (TMC435, a + simeprevir and RBV + daclatasvir (BMS-790052, an + daclatasvir and RBV + GS-5885 (an NS5a inhibitor) + GS-5885 and RBV First DAA study in people with HCC (pretransplant), split dosing (200 mg twice daily) |
|
IDX-184 50 mg, 100 mg Once-daily Idenix |
Phase II Genotype 1 |
Being studied + PEG-IFN/RBV |
|
mericitabine 500 mg, 1,000 mg, Twice-daily Hoffmann-La Roche |
Phase II Genotypes 1 and 4 Genotype 1b and 4 Genotypes 1 and 4 Genotype 1 Genotype 1 |
Being studied + PEG-IFN/RBV + danoprevir/r (ritonavir-boosted + danoprevir/r and RBV + danoprevir and PEG-IFN + danoprevir/r with PEG-IFN/RBV + telaprevir (protease inhibitor) + boceprevir (protease inhibitor) Protease inhibitor–experienced
|
TABLE 7. NS5a Inhibitors in Phase II and Phase III
Agent/Dose/Sponsor |
Phase/Population/Duration |
Comments |
|---|---|---|
|
ABT-267 5 mg, 50 mg, 200 mg Once-daily Abbott Laboratories
|
Phase II Genotypes 1, 2, and 3 Genotype 1 |
Being studied + PEG-IFN/RBV + ABT-450/r (ritonavir-boosted + ABT-450/r and RBV + ABT-333 (non-nucleoside + ABT-450/r and ABT-333 + ABT-450/r and ABT-333 with RBV (triple and quad therapy in treatment-naive and null responders) |
|
daclatasvir 60 mg Once-daily Bristol-Myers Squibb |
Phase II and Phase III Genotype 1 Genotype 1 Genotypes 1, 2, and 3 Genotype 4 Genotypes 1 and 4 |
Being studied + peginterferon lambda + peginterferon lambda and RBV + asunaprevir (BMS-650032, a + peginterferon lambda, with + PEG-IFN/RBV + GS-7977 (formerly PSI-7977, a + GS-7977 and RBV + BMS-791325 (non-nucleoside + BMS-791325 and asunaprevir + asunaprevir with PEG-IFN/RBV + BMS-986094 (formerly INX-189, + BMS-986094 and RBV Null- and partial responder quad trials not open as of 6/12 |
|
GS-5885 30 mg and 90 mg Once-daily Gilead Sciences |
Phase II Genotype 1 Genotype 1 Genotype 1 Genotype 1 Genotype 1 Genotype 1 |
Being studied + PEG-IFN/RBV + GS-9451 (a protease inhibitor) + GS-9451 and tegobuvir + GS-9451 and tegobuvir with RBV + GS-9451 and tegobuvir, with + GS-9451 with PEG-IFN/RBV + GS-7977 (formerly PSI-7977, a
|
|
GSK2336805 120 mg Once daily GlaxoSmithKline |
Phase II Genotypes 1 and 4 |
Being studied with PEG-IFN/RBV |
|
IDX-719 1 mg – 100 mg Once-daily Idenix |
Phase I and II Genotype 1
|
Single and multiple doses assessed |
TABLE 8. Protease Inhibitors in Phase II and Phase III
Agent/Dose/Sponsor |
Phase/Population/Duration |
Comments |
|---|---|---|
|
ABT-450/r 150 mg (boosted with 100 mg of ritonavir) Once-daily Abbott Laboratories |
Phase II Genotype 1 Genotype 1 Genotypes 1, 2, and 3 Genotype 1 Genotype 1 Up to 48 weeks |
Being studied + ABT-333 (non-nucleoside + ABT-333 and RBV + ABT-267 (NS5a inhibitor) + ABT-267 and RBV + ABT-267 and ABT-333 + ABT-333 and ABT-267 with RBV + ABT-267 and PEG-IFN/RBV Quad study not open as of 6/2012
|
|
ACH-1625 200 mg, 400 mg, Once-daily Achillion |
Phase II Genotype 1 |
Being studied with PEG-IFN/RBV |
|
asunaprevir 200 mg Once-daily Bristol-Myers Squibb |
Phase II and Phase III Genotype 1 Genotypes 1 and 4 Genotype 1b Genotypes 1, 2, 3, and 4 Genotypes 1 and 4 |
Being studied + peginterferon lambda + peginterferon lambda and RBV + daclatasvir (BMS-790052, an + daclatasvir and BMS-791325 + daclatasvir with peginterferon + daclatasvir with PEG-IFN/RBV Quad in null responders not open as of 6/12 |
|
BI 201335 120 mg, 240 mg Once-daily Boehringer Ingelheim |
Phase II and phase III Genotype 1 Genotype 1 Genotype 1 |
Being studied + PEG-IFN/RBV + BI 207127 (non-nucleoside + BI 207127 and RBV + BI 207127 with PEG-IFN/RBV Treatment-naive/relapse study not open as of 6/12
|
|
danoprevir/r 100 mg (boosted with 100 mg of ritonavir) Twice-daily; fixed-dose combination is in phase I Hoffmann-La Roche/Genentech |
Phase II Genotype 1 Genotypes 1 and 4 Genotypes 1 and 4 Genotype 1 Genotypes 1 and 4 Genotype 1 |
Being studied + PEG-IFN/RBV + mericitabine (RG7128, a + mericitabine and RBV + mericitabine with PEG-IFN/RBV Trial in protease inhibitor-experienced not open as of 6/12 |
|
GS-9256 75 mg, 150 mg Twice-daily Gilead Sciences |
Phase II Genotype 1 Genotype 1 |
Being studied + PEG-IFN/RBV + tegobuvir (GS-9190, a non- |
|
GS-9451 200 mg Once-daily Gilead Sciences |
Phase II Genotype 1 Genotype 1 Genotype 1 Genotype 1 Genotype 1 |
Being studied + PEG-IFN/RBV + tegobuvir (GS 9190, a non- + GS-5885 (NS5a inhibitor) + GS-5885 and RBV + GS-5885 and tegobuvir with RBV + GS-5885 with PEG-IFN/RBV + GS-5885 (with PEG-IFN/ RBV
|
|
MK-5172 Various doses studied, reduced to 100 mg/day 100 mg (800 mg in 7-day monotherapy trial only) Once-daily Merck
|
Phase I and phase II Genotype 1 Genotype 1 |
Being studied as monotherapy + PEG-IFN/RBV
|
|
simeprevir 150 mg (100 mg dose studied in Japan) Once-daily Janssen Pharmaceuticals/Tibotec/Medivir |
Phase II and phase III Genotype 1 Genotype 1 Genotype 4 Genotype 1 Genotype 1 Genotype 1 |
Being studied + PEG-IFN/RBV + GS-7977 (formerly PSI-7977, a + GS-7977 and RBV
|
|
vaniprevir 300 mg Merck Twice-daily |
Phase III |
Being developed in Japan only |
TABLE 9. Non-Nucleoside Polymerase Inhibitors in Phase II and Phase III
Agent/Dose/Sponsor |
Phase/Population/Duration |
Comments |
|---|---|---|
|
ABT-072 400 mg Once-daily Abbott Laboratories |
Phase II |
No new studies listed |
|
ABT-333 400 mg Twice-daily Abbott Laboratories |
Phase II Genotype 1 Genotype 1 Genotype 1 |
Being studied + ABT-450/r (ritonavir-boosted + ABT-450/r and ABT-267 + ABT-450/r and ABT-267 with
|
|
BI 207127 600 mg Twice-daily Boehringer Ingelheim |
Phase II Genotype 1 |
Being studied + BI 201335 (protease inhibitor) + BI 201335 and RBV + BI 201335 with PEG-IFN/RBV |
|
BMS-791325 75 mg, 150 mg Twice-daily Bristol-Myers Squibb |
Phase II Genotype 1 Genotype 1 |
Being studied + PEG-IFN/RBV + asunaprevir (BMS-650032, |
|
setrobuvir 200 mg Twice-daily Anadys/ |
Phase II Genotype 1 |
Being studied with PEG-IFN/RBV |
|
tegobuvir 20 mg, 30 mg, 40 mg Twice-daily Gilead Sciences |
Phase II Genotype 1 Genotype 1 Genotype 1 |
Being studied + GS-9526 (protease inhibitor) + GS-9451 (protease inhibitor) + GS-5885 (NS5a inhibitor) and + GS-5885 and GS-9451 with |
|
VX-222 400 mg Twice-daily (coadministered with 1125 mg of telaprevir twice-daily) Vertex Pharmaceuticals |
Phase II Genotype 1a only Genotype 1 Genotype 1 |
Being studied + telaprevir (protease inhibitor) + telaprevir with PEG-IFN/RBV Study in Genotype 1a not open as of 6/12 |
References
1. Perz JF, Armstrong GL, Farrington LA, et al. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006 Oct;45(4):529–38.
2. Morgan TR, Ghany MG, Kim HY, et al.; HALT-C Trial Group. Outcome of sustained virological responders with histologically advanced chronic hepatitis C. Hepatology. 2010 Sep;52(3):833–44.
3. Ng V, Saab S. Effects of a sustained virologic response on outcomes of patients with chronic hepatitis C. Clin Gastroenterol Hepatol. 2011 Nov;9(11):923–30.
4. Singal AK, Singh A, Jaganmohan S, et al. Antiviral therapy reduces risk of hepatocellular carcinoma in patients with hepatitis C virus-related cirrhosis. Clin Gastroenterol Hepatol. 2010 Feb;8(2):192–9.
5. Singal AG, Volk ML, Jensen D, et al. A sustained viral response is associated with reduced liver-related morbidity and mortality in patients with hepatitis C virus. Clin Gastroenterol Hepatol. 2010 Mar;8(3):280–8, 288.e1.
6. Lavanchy D. Evolving epidemiology of hepatitis C virus. Clin Microbiol Infect. 2011 Feb;17(2):107–15.
7. Dienstag JL, Ghany MG, Morgan TR, et al.; HALT-C Trial Group. A prospective study of the rate of progression in compensated, histologically advanced chronic hepatitis C. Hepatology. 2011 Aug;54(2):396–405.
8. Ly KN, Xing J, Klevens RM, et al. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann Intern Med. 2012 Feb 21;156(4):271–8.
9. Bacon BR, Gordon SC, Lawitz E, et al.; HCV RESPOND-2 Investigators. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011 Mar 31;364(13):1207–17.
10. Jacobson IM, McHutchison JG, Dusheiko G, et al.; ADVANCE Study Team. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011 Jun 23;364(25):2405–16.
11. Poordad F, McCone J Jr, Bacon BR, et al.; SPRINT-2 Investigators. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011 Mar 31;364(13):1195–206.
12. Sherman KE, Flamm SL, Afdhal NH, et al.; ILLUMINATE Study Team. Response-guided telaprevir combination treatment for hepatitis C virus infection. N Engl J Med. 2011 Sep 15;365(11):1014–24.
13. Zeuzem S, Andreone P, Pol S, et al.; REALIZE Study Team. Telaprevir for retreatment of HCV infection. N Engl J Med. 2011 Jun 23;364(25):2417–28.
14. Hézode C, Dorival C, Zoulim F, et al. Safety of telaprevir or boceprevir in combination with peginterferon alfa/ribavirin, in cirrhotic non responders. First results of the French early access program (ANRS CO20-CUPIC) (Abstract 8). Paper presented at: 47th Annual Meeting of the European Association for the Study of the Liver; 2012 April 18–22; Barcelona, Spain. Available from: http://mobile.ilcapp.eu/EASL_161/poster_23756/program.aspx. (Accessed 2012 June 25)
15. Bruggmann P, Falcato L, Dober S, et al.; Swiss Hepatitis C Cohort Study. Active intravenous drug use during chronic hepatitis C therapy does not reduce sustained virological response rates in adherent patients. J Viral Hepat. 2008 Oct;15(10):747–52.
16. Grebely J, Matthews GV, Hellard M, et al.; ATAHC Study Group. Adherence to treatment for recently acquired hepatitis C virus (HCV) infection among injecting drug users. J Hepatol. 2011 Jul;55(1):76–85.
17. Hellard M, Sacks-Davis R, Gold J. Hepatitis C treatment for injection drug users: a review of the available evidence. Clin Infect Dis. 2009 Aug 15;49(4):561–73.
18. Robaeys G, Matheï C, Buntinx F, et al. Management of hepatitis C virus infections in intravenous drug users. Acta Gastroenterol Belg. 2002 Apr–Jun;65(2):99–100.
19. Sylvestre DL, Clements BJ. Adherence to hepatitis C treatment in recovering heroin users maintained on methadone. Eur J Gastroenterol Hepatol. 2007 Sep;19(9):741–7.
20. Taylor LE. Delivering care to injection drug users coinfected with HIV and hepatitis C virus. Clin Infect Dis. 2005 Apr 15;40 Suppl 5:S355–61.
21. Chayama K, Takahashi S, Kawakami Y, et al. Dual oral combination therapy with the NS5A inhibitor BMS-790052 and the NS3 protease inhibitor BMS-650032 achieved 90% sustained virologic response (SVR12) in HCV genotype 1b-infected null responders (Abstract LB-4). Paper presented at: 62nd Annual Meeting of the American Association for the Study of Liver Disease; 2011 November 4–8; San Francisco, CA.
22. Suzuki F, Ikeda K, Toyota J, et al. Dual oral therapy with the NS5a inhibitor daclatasvir (BMS 790052) and NS3 protease inhibitor asunaprevir (BMS 650032) in HCV genotype 1b-infected null responders or patients ineligible/intolerant to peginterferon/ribavirin (Abstract 2344). Paper presented at: 47th Annual Meeting of the European Association for the Study of the Liver; 2012 April 18–22; Barcelona, Spain. Available from: http://mobile.ilcapp.eu/EASL_161/poster_23762/program.aspx.
23. Lok A, Gardiner D, Hézode C, et al. Confirmation that quadruple therapy with daclatasvir (NS5a inhibitor), asunaprevir (NS3 inhibitor), and peginterferon/ribavirin results in high rate of SVR4 in HCV genotype1 null responders (Abstract 1415). Poster session presented at: 47th Annual Meeting of the European Association for the Study of the Liver; 2012 April 18–22; Barcelona, Spain. Available from: http://mobile.ilcapp.eu/EASL_161/poster_24851/program.aspx. (Accessed 2012 June 25)
24. Marzolini C, Elzi L, Gibbons S, et al.; Swiss HIV Cohort Study. Prevalence of comedications and effect of potential drug-drug interactions in the Swiss HIV Cohort Study. Antivir Ther. 2010;15(3):413–23.
25. Marzolini C, Back D, Weber R, et al.; Swiss HIV Cohort Study Members. Ageing with HIV: medication use and risk for potential drug-drug interactions. J Antimicrob Chemother. 2011 Sep;66(9):2107–11.
26. Kiser JJ, Burton JR, Anderson PL, et al. Review and management of drug interactions with boceprevir and telaprevir. Hepatology. 2012 May;55(5):1620–8.
27. Fried M, Buti M, Dore GJ, et al. TMC435 in combination with peginterferon and ribavirin in treatment-naive HCV genotype 1 patients: final analysis of the PILLAR phase IIb study (Abstract LB-5). 62nd Annual Meeting of the American Association for the Study of Liver Diseases; 2011 November 4–8; San Francisco, CA.
28. Zeuzem S, Berg T, Gane E, et al. TMC435 in HCV genotype 1 patients who have failed previous pegylated interferon/ribavirin treatment: final SVR24 results of the ASPIRE trial. 47th Annual Meeting of the European Association for the Study of the Liver; 2012 April 18–22; Barcelona, Spain. Abstract 2. Available from: http://mobile.ilcapp.eu/EASL_161/poster_23749/program.aspx. (Accessed 2012 June 25)
29. Lenz O, Fevery B, Vijgen L, et al. TMC435 in patients infected with HCV genotype 1 who have failed previous pegylated interferon/ribavirin treatment: virologic analysis of the ASPIRE trial. 47th Annual Meeting of the European Association for the Study of the Liver; 2012 April 18–22; Barcelona, Spain. Abstract 9. Available from: http://mobile.ilcapp.eu/EASL_161/session_2527/program.aspx#23757. (Accessed 2012 June 25)
30. Sulkowski M, Emanoil C, Asselah T, et al. Sustained Virologic Response (SVR) and safety of BI201335 combined with peginterferon alfa-2a and ribavirin (P/R) in treatment-naive patients with chronic genotype 1 HCV infection. 46th International Liver Congress; 2011 March 30–April 3; Berlin, Germany. Abstract 60/324.
31. Sulkowski M, Bourliere M, Bronowicki J-P, et al. SILEN-C2: Sustained Virologic Response (SVR) and safety of BI201335 combined with peginterferon alfa-2a and ribavirin (P/R) in chronic HCV genotype-1 patients with non-response to P/R (Abstract 66/330). 46th Annual Meeting of the European Association for the Study of the Liver; 2011 March 30–April 3; Berlin, Germany.
32. Dieterich D, Asselah T, Guyader D, et al. SILEN-C3: Treatment for 12 or 24 weeks with BI201335 combined with peginterferon alfa-2a and ribavirin (P/R) in treatment-naïve patients with chronic genotype-1 HCV infection (Abstract 36). 62nd Annual Meeting of the American Association for the Study of Liver Diseases; 2011 November 4–8; San Francisco, CA.
33. Lok AS, Gardiner DF, Lawitz E, et al. Preliminary study of two antiviral agents for hepatitis C genotype 1. N Engl J Med. 2012 Jan 19;366(3):216–24.
34. Kowdley K, Lawitz E, Crespo I, et al. GS 7977 + PEG/RBV in HCV genotype 1: The ATOMIC Trial. An end to response-guided therapy? (Abstract 1). Paper presented at: 47th Annual Meeting of the European Association for the Study of the Liver; 2012 April 18–22; Barcelona, Spain. Available from: http://mobile.ilcapp.eu/EASL_161/poster_23748/program.aspx. (Accessed 2012 June 25)
35. Everson G, Cooper C, Shiffman ML, et al. Rapid and sustained achievement of undetectable HCV RNA during treatment with ritonavir-boosted danoprevir/PEG-IFNa-2A/RBV in HCV genotype 1 or 4 patients: Dauphine week 36 interim analysis (Abstract 1177). Paper presented at: 47th Annual Meeting of the European Association for the Study of the Liver; 2012 April 18–22; Barcelona, Spain. Available from: http://mobile.ilcapp.eu/EASL_161/poster_24544/program.aspx. (Accessed 2012 June 25)
36. Zeuzem S, Arora S, Bacon B, et al.; EMERGE Study Group. Peginterferon lambda-1-a (Lambda) compared to peginterferon alfa-2a (alpha) in treatment-naïve patients with HCV genotypes (G) 2 or 3: first SVR24 results from Emerge phase IIB (Abstract 10). Paper presented at: 47th Annual Meeting of the European Association for the Study of the Liver; 2012 April 18–22; Barcelona, Spain. Available from: http://mobile.ilcapp.eu/EASL_161/poster_23758/program.aspx. (Accessed 2012 June 25).
37. Di Bisceglie, A Sulkowski M, Gane E, et al. VX-222/telaprevir in combination with peginterferon-alfa-2A and ribavirin in treatment-naïve genotype 1 HCV patients treated for 12 weeks: ZENITH study, SVR12 interim analysis (Abstract A020). Paper presented at: Canadian Digestive Diseases Weekend and the Annual CDSL Winter Meeting. 2012 February 24–27; Montreal, Québec. Available from: http://www.cag-acg.org/uploads/2012_abstracts_final_web.pdf. (Accessed 2012 June 25)
38. Zeuzem S, Buggisch P, Agarwal K, et al. Dual, triple, and quadruple combination treatment with a protease inhibitor (GS-9256) and a polymerase inhibitor (GS-9190) alone and in combination with ribavirin (RBV) or PegIFN/RBV for up to 28 days in treatment naïve, genotype 1 HCV subjects (Abstract LB-1). 61st Annual Meeting of the American Association for the Study of Liver Diseases; 2010 October 29–November 2; Boston, MA.
39. Gane EJ, Stedman C, Anderson J, et al. 100% rapid virologic response for PSI-7977 + ribavirin in genotype 1 null responders (ELECTRON): early viral decline similar to that observed in genotype 1 and genotype 2/3 treatment-naive patients (Abstract 54LB). Paper presented at: 19th Conference on Retroviruses and Opportunistic Infections; 2012 March 5–8; Seattle, WA. Available from: http://www.retroconference.org/2012b/Abstracts/45476.htm. (Accessed 2012 June 25)
40. Gane EJ, Stedman CA, Hyland RH, et al. ELECTRON: once daily PSI-7977 plus RBV in HCV GT1/2/3 (Abstract 1113). Paper presented at: 47th Annual Meeting of the European Association for the Study of the Liver; 2012 April 18–22; Barcelona, Spain. Available from: http://mobile.ilcapp.eu/EASL_161/poster_24480/program.aspx. (Accessed 2012 June 25)
41. Lawitz E, Poordad F, Kowdley KV, et al. A 12-week interferon-free regimen of ABT-450r, ABT-072, and ribavirin was well tolerated and achieved sustained virological response in 91% of treatment-naïve HCV IL28B-CC genotype-1-infected patients (Abstract 13). Paper presented at: 47th Annual Meeting of the European Association for the Study of the Liver; 2012 April 18–22; Barcelona, Spain. Available from: http://mobile.ilcapp.eu/EASL_161/poster_23761/program.aspx. (Accessed 2012 June 25)
42. Pearlman BL, Traub N. Sustained virologic response to antiviral therapy for chronic hepatitis C virus infection: a cure and so much more. Clin Infect Dis. 2011 Apr 1;52(7):889–900.
43. Pradat P, Tillmann HL, Sauleda S, et al.; HENCORE Group. Long-term follow-up of the hepatitis C HENCORE cohort: response to therapy and occurrence of liver-related complications. J Viral Hepat. 2007;14:556–63.
44. Swain MG, Lai MY, Shiffman ML, et al. A sustained virologic response is durable in patients with chronic hepatitis C treated with peginterferon alfa-2a and ribavirin. Gastroenterology. 2010;139:1593–601.
45. Veldt BJ, Saracco G, Boyer N, et al. Long term clinical outcome of chronic hepatitis C patients with sustained virological response to interferon monotherapy. Gut. 2004;53:1504–8.
46. Sulkowski M, Gardiner D, Lawitz E, et al. Potent viral suppression with all-oral combination of daclatasvir (NS5A inhibitor) and GS-7977 (NS5B inhibitor), +/-ribavirin, in treatment-naïve patients with chronic HCV GT1, 2, or 3 (Abstract 1422). Poster session presented at: 47th Annual Meeting of the European Association for the Study of the Liver; 2012 April 18–22; Barcelona, Spain. Available from: http://mobile.ilcapp.eu/EASL_161/poster_24858/program.aspx. (Accessed 2012 June 25)
47. Lawitz E, Rodriguez-Torres M, Cornpropst J, et al. The effect of hepatic impairment on the safety, pharmacokinetics and antiviral activity of GS-7977 in hepatitis-C infected subjects treated for seven days (Abstract 1130). Paper presented at: 47th Annual Meeting of the European Association for the Study of the Liver; 2012 April 18–22; Barcelona, Spain. Available from: http://mobile.ilcapp.eu/EASL_161/poster_24497/program.aspx. (Accessed 2012 June 25)
48. Gilead Sciences (Press Release). Gilead announces early sustained virologic response rates for GS-7977 plus ribavirin in genotype 1 treatment naive hepatitis C patients-Interim results reported from ELECTRON and QUANTUM studies. 2012 April 18. Available from: http://www.gilead.com/pr_1684792. (Accessed 2012 June 25)
49. Poordad F, Lawitz E, Kowdley KV, et al. 12-week interferon-free regimen of ABT-450/r + ABT-333 + ribavirin achieved SVR12 in more than 90% of treatment-naïve HCV genotype-1-infected subjects and 47% of previous non-responders (Abstract 1399). Paper presented at: 47th Annual Meeting of the European Association for the Study of the Liver; 2012 April 18–22; Barcelona, Spain. Abstract 1399. Available from: http://mobile.ilcapp.eu/EASL_161/poster_24791/program.aspx. (Accessed 2012 June 25)
50. Zeuzem S, Soriano V, Asselah T, et al. SVR4 and SVR12 with an interferon-free regimen of BI201335 and BI207127, +/- ribavirin, in treatment-naïve patients with chronic genotype-1 HCV infection: interim results of SOUND-C2 (Abstract 101). Paper presented at: 47th Annual Meeting of the European Association for the Study of the Liver; 2012 April 18–22; Barcelona, Spain. Available from: http://mobile.ilcapp.eu/EASL_161/poster_24354/program.aspx. (Accessed 2012 June 25)
51. Manns MP, Bourlière M, Benhamou Y, et al. Potency, safety, and pharmacokinetics of the NS3/4A protease inhibitor BI201335 in patients with chronic HCV genotype-1 infection. J Hepatol. 2011 Jun;54(6):1114–22.
52. Soriano V, Gane E, Angus P, et al. The efficacy and safety of the interferon-free combination of BI201335 and BI207127 in genotype 1 HCV patients with cirrhosis: interim analysis from SOUND-C2 (Abstract 1420). Poster session presented at: 47th Annual Meeting of the European Association for the Study of the Liver; 2012 April 18–22; Barcelona, Spain. Available from: http://mobile.ilcapp.eu/EASL_161/poster_24856/program.aspx. (Accessed 2012 June 25)
53. Gane EJ, Pockros P, Zeuzem S, et al. Interferon-free treatment with combination of mericitabine and danoprevir/r with or without ribavirin in treatment-naïve HCV genotype-1 infected patients (Abstract 1412). 47th Annual Meeting of the European Association for the Study of the Liver; 2012 April 18–22; Barcelona, Spain. Available from: http://mobile.ilcapp.eu/EASL_161/poster_24848/program.aspx. (Accessed 2012 June 25)
54. Alter MJ. Epidemiology of viral hepatitis and HIV co-infection. J Hepatol. 2006;44(1 Suppl):S6–9.
55. Chen TY, Ding EL, Seage Iii GR, et al. Meta-analysis: increased mortality associated with hepatitis C in HIV-infected persons is unrelated to HIV disease progression. Clin Infect Dis. 2009 Nov 15;49(10):1605–15.
56. Lewden C, Salmon D, Morlat P, et al.; Mortality 2000 study group. Causes of death among human immunodeficiency virus (HIV)-infected adults in the era of potent antiretroviral therapy: emerging role of hepatitis and cancers, persistent role of AIDS. Int J Epidemiol. 2005 Feb;34(1):121–30.
57. Martínez E, Milinkovic A, Buira E, et al. Incidence and causes of death in HIV-infected persons receiving highly active antiretroviral therapy compared with estimates for the general population of similar age and from the same geographical area. HIV Med. 2007 May 8;(4):251–8.
58. Neuhaus J, Angus B, Kowalska JD, et al.; INSIGHT SMART and ESPRIT study groups. Risk of all-cause mortality associated with nonfatal AIDS and serious non-AIDS events among adults infected with HIV. AIDS. 2010 Mar 13;24(5):697–706.
59. Palella FJ Jr, Baker RK, Moorman AC, et al.; HIV Outpatient Study Investigators. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006 Sep;43(1):27–34.
60. Smith C, Sabin CA, Lundgren JD, et al.; Data Collection on Adverse Events of Anti-HIV drugs (D:A:D) Study Group. Factors associated with specific causes of death amongst HIV-positive individuals in the D:A:D study. AIDS. 2010 Jun 19;24(10):1537–48.
61. Berenguer J, Alvarez-Pellicer J, Martín PM, et al.; GESIDA3603/5607 Study Group. Sustained virological response to interferon plus ribavirin reduces liver-related complications and mortality in patients coinfected with human immunodeficiency virus and hepatitis C virus. Hepatology. 2009 Aug;50(2):407–13.
62. Berenguer J, Rodríguez E, Miralles P, et al.; the GESIDA HIV/HCV Cohort Study Group. Sustained virological response to interferon plus ribavirin reduces non-liver-related mortality in patients coinfected with human immunodeficiency virus and hepatitis C virus. Clin Infect Dis. 2012 May 18. [Epub ahead of print]
63. U.S. Department of Health and Human Services; Food and Drug Administration (U.S.). Center for Drug Evaluation and Research (CDER). Guidance for Industry. Chronic Hepatitis C Virus Infection: Developing Direct-Acting Antiviral Agents for Treatment. September 2010. Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM225333.pdf. (Accessed 2012 June 25)
64. Dieterich D Soriano V, Sherman K, et al.; Study 110 Team. Telaprevir in Combination with Pegylated Interferon-alfa-2a+RBV in HCV/HIV-co-infected Patients: A 24-Week Treatment Interim Analysis (Abstract 46). Paper presented at: 19th Conference on Retroviruses and Opportunistic Infections; 2012 March 5–8; Seattle, WA. Available from: http://www.retroconference.org/2012b/Abstracts/42969.htm. (Accessed 2012 June 25)
65. Sulkowski M, Pol S, Cooper C, et al. Boceprevir + pegylated interferon + ribavirin for the treatment of HCV/HIV-co-infected patients: end of treatment (week-48) interim results (Abstract 47). Paper presented at: 19th Conference on Retroviruses and Opportunistic Infections; 2012 March 5–8; Seattle, WA. Available from: http://www.retroconference.org/2012b/Abstracts/44725.htm. (Accessed 2012 June 25)
66. Department of Health and Human Services. Office of the Assistant Secretary for Planning and Evaluation (ASPE). ASPE Issue Brief. Overview of the uninsured in the United States: A summary of the 2011 current population survey. September 13, 2011. Available from: http://aspe.hhs.gov/health/reports/2011/CPSHealthIns2011/ib.pdf. (Accessed 2012 June 25)
