Updates to this chapter can be found in the postscript at the end of this page.
Original Report July 2012
By Erica Lessem
Introduction
Tuberculosis (TB) persists as a global health problem, suffering from both insufficient funding and political will. As a result of poor treatment options and inadequate administration of care, increasingly intractable drug-resistant (DR) strains of the disease are developing.[1],[2] Nevertheless, major advances in the fight against TB in the past year inspire optimism.
Implementers, activists, policy makers, and researchers met last month in Cambridge, Massachusetts, to begin discussions about how to reset the global community’s target to zero new TB infections, zero TB deaths, and zero TB suffering and stigma.
Founded in fall 2011, the Global TB Community Advisory Board, a group of activists from around the world who are extensively involved in HIV and TB research networks, convened to increase community involvement in tuberculosis research and to mobilize political will regarding key TB product-development issues.
Several community and household-level intervention studies demonstrated the importance of case finding and treatment of latent TB infections, particularly among people with HIV. The World Health Organization (WHO) issued guidelines to integrate TB and HIV service delivery, and many studies are being done to examine the drug-drug interactions (DDIs) between commonly used antiretrovirals (ARVs) and new and existing TB drugs.[3]
The TB Alliance helped revitalize the traditionally slow and inefficient drug-development paradigm by presenting promising results of the first preliminary study of a novel combination treatment regimen, and by initiating the first trial to study both drug-sensitive TB (DS-TB) and multidrug-resistant TB (MDR-TB, or TB that is resistant to at least isoniazid and rifampicin) together, using the same treatment for both.
At the end of 2011, Otsuka filed for European Medicines Agency (EMA) approval for its new drug delamanid (OPC-67683) for treatment of drug- resistant TB—the first new drug and new class of drugs submitted for approval to a stringent regulatory authority in 40 years. Including delamanid, six new drug candidates from four different classes are in mid-stage clinical trials for TB. Finally, on July 2, 2012, Janssen Therapeutics announced its filing a New Drug Application (NDA) for bedaquiline (TMC207) with the U.S. Food and Drug Administration (FDA) for treatment of drug-resistant TB.[4]
LATENT TB INFECTION
One-third of the world is estimated to have latent TB infection (LTBI), making over 2 billion people potential future TB cases. Treating LTBI is essential to reaching zero new infections from TB. Many recent studies aim to optimize LTBI treatment, either by adding new drugs to shorten treatment duration or by conducting operational research to maximize the benefits of the current standard of care, 6–12 months of daily isoniazid preventive therapy (IPT). Research is also beginning to tackle LTBI in the contacts of DR cases. Options for preventive therapy of DR-TB are essential, especially as a recent study in India found that contacts of isoniazid-resistant TB patients are more likely to be infected than are contacts of isoniazid-susceptible cases (although incidence of TB disease was similar in both groups).[5]
High-burden countries are recognizing the importance of treating LTBI. For example, Botswana’s efforts in the past decade to ramp up IPT resulted in the enrollment of 72,000 eligible patients between 2005 and 2007. Mozambique increased the number of HIV patients receiving IPT almost twentyfold from 2008 to 2010. South Africa’s ambitious new National Strategic Plan, rolled out in December 2011, has a long-term vision that includes zero TB and HIV infections, and calls for all South Africans to be screened and tested for TB at least once yearly. Many countries, however, still require IPT scale-up. In particular, to conform with the WHO Policy on Collaborative TB/HIV Activities, IPT scale-up for people (especially women and children) coinfected with HIV is necessary.[6]
TABLE 1. Latent Tuberculosis Infection (LTBI) Studies as of June 2012
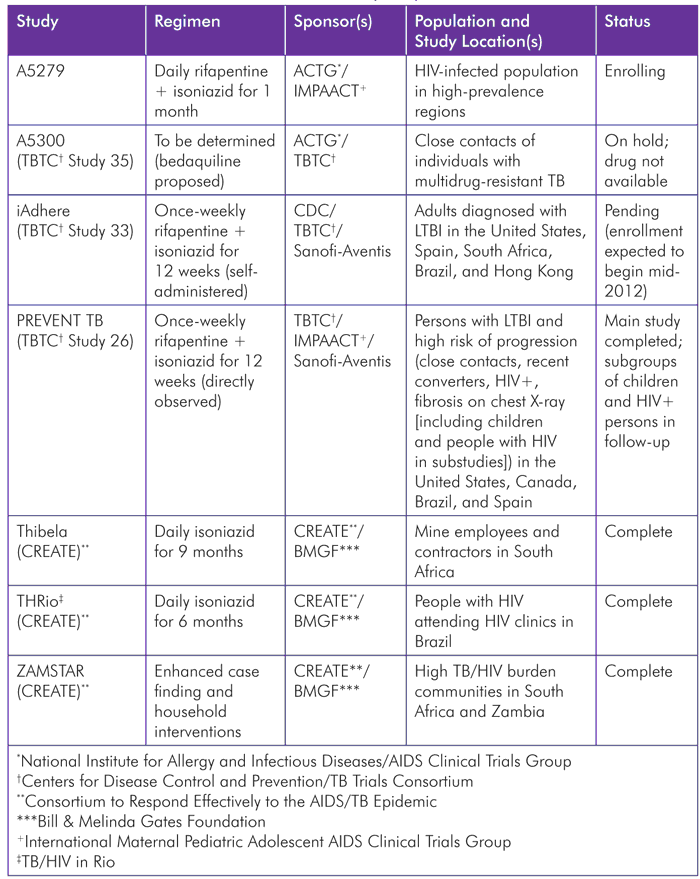
The TB Trials Consortium (TBTC), an international research network funded by the U.S. Centers for Disease Control and Prevention (CDC), completed PREVENT TB, a study with Sanofi-Aventis to evaluate rifapentine in treatment-shortening regimens for LTBI and active disease. PREVENT TB, or TBTC Study 26, determined that 12 weeks of once-weekly isoniazid and rifapentine under directly observed therapy were as effective as the standard, self-administered nine months of daily isoniazid. Moreover, patients in the 12-week arm had higher completion rates and less liver toxicity.[7] These promising outcomes resulted in a recommendation by the CDC in December 2011 of the 12-week regimen of once-weekly isoniazid and rifapentine under direct observation.[8] Results of substudies including children and people living with HIV show the regimen to be well tolerated in these populations; those results will be published in 2012.[9] Sanofi-Aventis is working on both a fixed-dose combination (FDC) and a dispersible form to facilitate the regimen’s administration in various settings and to children.[10]
As directly observed therapy is not ideal for all programs and patients, the TBTC is also planning the iAdhere study (Study 33) to evaluate adherence to this new regimen given self-administered versus with directly observed therapy. The study will have two self-administered arms, one with and one without text-message reminders to patients to take their medication.[11] Results of this phase IV study should help optimize the use of the 12-week isoniazid and rifapentine regimen in TB programs worldwide.
The AIDS Clinical Trials Group (ACTG) and the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT)—two research networks funded by the Division of AIDS, National Institute of Allergy and Infectious Diseases (NIAID) of the U.S. National Institutes of Health (NIH)—are building upon the treatment-shortening success of rifapentine and isoniazid with their plans to initiate a one-month regimen of daily rifapentine and isoniazid to treat latent TB in HIV-infected individuals. Study A5279, now enrolling, will contribute to dramatically shortening the time of LTBI treatment if the regimen proves successful.[12]
The ACTG, TBTC, and IMPAACT are also planning ACTG Study A5300 (also known as TBTC Study 35) to examine regimens for preventing TB disease in those 13 years and older who have household contact with persons with confirmed DR-TB. Originally, this study planned to evaluate the efficacy and tolerability of the new compound bedaquiline compared with isoniazid, but bedaquiline’s sponsor, Janssen Infectious Diseases, is waiting for additional experience with the drug in patients before making it available for use in LTBI studies. The study design team is now looking to animal-model studies at the Johns Hopkins University to determine the best available drugs to use for DR-TB prophylaxis in place of bedaquiline.[13] A clinical trial for DR-TB prophylaxis would constitutea critical step toward reducing DR-TB incidence.
Scientific and regulatory challenges in therapeutic clinical trials
Several scientific and regulatory challenges threaten the field of TB clinical research. The endpoints for phase II and phase III studies are controversial and complicated. Follow-up for relapse often requires two years and multiple patient visits. Particularly in the case of MDR-TB and XDR-TB trials, where treatment with optimized background regimens can take years, these lengthy and cumbersome follow-up periods hinder timely drug development. To make research more efficient and enable drugs to move more quickly through the development pipeline, increased research into biomarkers and other predictors of cure and relapse is urgently needed.
Promisingly, based on its use of symptomatic endpoints for clinical trials for the treatment of community-acquired bacterial pneumonia, the U.S. Food and Drug Administration has also expressed openness to considering full approval for TB treatment based on a combined microbiological endpoint (e.g., durable culture conversion) plus a combined symptomatic endpoint, while requiring clinical follow-up for relapse.[14] As all patients in this trial design would contribute data (time to culture conversion, and time to symptom resolution) for the approval endpoint, the pivotal study would not need to be powered to detect statistically significant differences in relapse rate, so long as relapse rates followed the same direction as the clinical and microbiological data. Thus, the acceptance of microbiological and symptomatic endpoints could be a game-changer in TB treatment trials by allowing them to be smaller and less expensive.
Another scientific challenge is the study of close contacts of MDR-TB cases. As MDR-TB is, by definition, resistant to isoniazid—the recommended drug for treating LTBI—IPT would not be effective in this population. However, it is unclear what drug(s) would be effective as standard treatment for LTBI in contacts of MDR-TB cases, particularly as some cases may have resistance to drugs in addition to isoniazid and rifampicin. These scientific challenges are compounded by an understandable reluctance of drug sponsors to administer investigational drugs to healthy patients with LTBI without full evidence of safety from testing in sick trial participants.
In addition to these studies of new regimens for treating LTBI, data are also available from three important studies on implementation of IPT and other methods to reduce TB incidence and prevalence. The Consortium to Respond Effectively to the AIDS/TB Epidemic (CREATE)—a group of research institutions based in Brazil, South Africa, the United States, and Zambia—led these studies.
The Thibela study, the largest IPT trial ever, examined whether community-wide administration of IPT could affect TB incidence in South African mines, where it is extremely high. This cluster-randomized trial enrolled 27,000 employees and contractors in 15 mine shafts. Those in clusters in the intervention arm received community education and TB screening, and if they did not have active TB, nine months of IPT and monitoring. Workforces in the control arm received the usual TB control program activities at their site. The results of Thibela showed that IPT has a dramatic impact on preventing TB in individuals while they are on therapy; however, the intervention did not durably reduce TB incidence at the community level.[15],[16]
The THRio (TB/HIV in Rio) study examined an IPT training and education intervention in 29 HIV clinics in Rio de Janeiro, Brazil, with 18,000 HIV-infected clients. This study compared incidence of active TB and mortality in clinics where patients received the IPT intervention with incidence in clinics where they had not yet received it, with all 29 clinics phased into the intervention by the study’s conclusion. Results indicate that IPT implementation can reduce TB incidence and death in regions with high TB and HIV burdens.[17]
The contrasting results between Thibela and THRio may be attributable to the unique population in the former. Both silicosis (a lung disease caused by inhalation of silica dust) and HIV infection are relatively common among miners in South Africa, and both increase the risk of TB. The Thibela research team was not allowed access to participants’ HIV status; however, HIV status was unlikely to be a confounder as the intervention and control clusters were balanced on all other variables (including self-reported HIV status). Additionally, Thibela’s results showed that the effect at the individual level of preventing TB is not durable once participants stop taking IPT. Finally, it was challenging to get entire communities at a time on IPT; however, even when 100% uptake was reached and a population-level effect was seen, the effect waned rapidly. Other reasons for lack of effect may be high ongoing rates of TB transmission and vulnerability due to HIV and silicosis. Modeling based on data of participants leaving and reentering their mining community will shed light on whether migration in these communities allows for re-exposure and introduction of TB infection.[18],[19],[20]
The ZAMSTAR study, one of the largest community-randomized trials, looked at the impact of different interventions on TB prevalence in Zambia and South Africa. Enhanced case-finding interventions, including widespread access to sputum smear microscopy outside of regular health services, did not seem to affect TB prevalence. However, interventions (the evaluation of household contacts of TB patients, with counseling, HIV testing, TB testing, and referral to services) in the homes of patients with TB in high TB/HIV-burden communities did show a nonsignificant trend in reducing the prevalence of culture-positive TB by 22% compared to communities without the intervention. Moreover, children living in the household-intervention communities were half as likely to become infected with TB as their counterparts in the control communities.[21] These results have now been taken up widely by the South African and Zambian health authorities, as well as the 15-country Southern African Development Community (SADC).[22],[23] The WHO also advocates for the use of widespread IPT in its policy on collaborative TB/HIV activities.[24]
Maternal TB
TB is estimated to cause 6–15% of all maternal mortality, and is one of the top three overall causes of death among women ages 15–45.[25],[26],[27] Genital TB causes between 1% and 16% of overall infertility, and results in a low chance of conception even after successful diagnosis and treatment.[28] As a result, it also causes painful stigma and social consequences for women. In addition, recent pregnancy is a demonstrated risk factor in developing active TB in women with HIV.[29],[30]
TB in pregnancy affects the mother: complications of TB and the need for prolonged treatment lead to increased maternal morbidity and mortality, according to a recent non-systematic review of the implications of maternal TB on obstetric and perinatal outcomes in South Asia.[31] Maternal TB also jeopardizes existing pregnancy, increasing the likelihood of spontaneous abortion, suboptimal weight gain, preterm labor, and the rare transmission of congenital TB.[32] It also affects the newborn, who has an increased risk of neonatal and perinatal mortality, low birth weight, and contracting postnatal TB.[33],[34] These risks increase further when mothers are diagnosed late, have advanced disease, and have incomplete or irregular drug treatment, indicating once more the urgency of early and appropriate TB treatment.[35] Proper care of maternal TB can also help reduce mother-to-child transmission of infections.[36]
The WHO and other leading international health organizations recommend the use of first-line drugs to treat DS-TB in pregnancy and while breastfeeding (although streptomycin should not be used during pregnancy).[37],[38] IPT is recommended for pregnant women with LTBI who are at risk of developing active disease.[39] Second-line drugs to treat DR-TB have been shown to produce good outcomes for mothers and their children, although there is a need for increased consideration of pregnant women in drug research.[40]
Because early diagnosis and adequate treatment are crucial for women’s health and the health of their children, it is critical to integrate early TB screening, prevention, and treatment into reproductive and other health programs. This is especially important in low-income countries, where an estimated 300,000 pregnant women are triply infected with TB, malaria, and HIV, causing severe complications in their pregnancies.[41] Implementing simple symptom screenings for TB, and prompt treatment for latent and active TB when indicated, at PEPFAR and other program sites preventing mother-to-child HIV transmission, and nutritional programs, can save the lives of many women and children.
ACTIVE TB DISEASE
Like LTBI, active TB disease is curable, yet lengthy treatment duration, high pill burdens, adverse effects, and under-resourced TB programs lead to poor adherence and cure rates. This in turn contributes to the development of DR-TB, whose treatment regimen is more difficult, lengthy, and expensive. Fortunately, researchers are working to improve TB treatment for both DS- and DR-TB by optimizing the use of existing drugs, and by studying novel compounds and combinations.
REPURPOSING EXISTING COMPOUNDS TO TREAT ACTIVE TB DISEASE
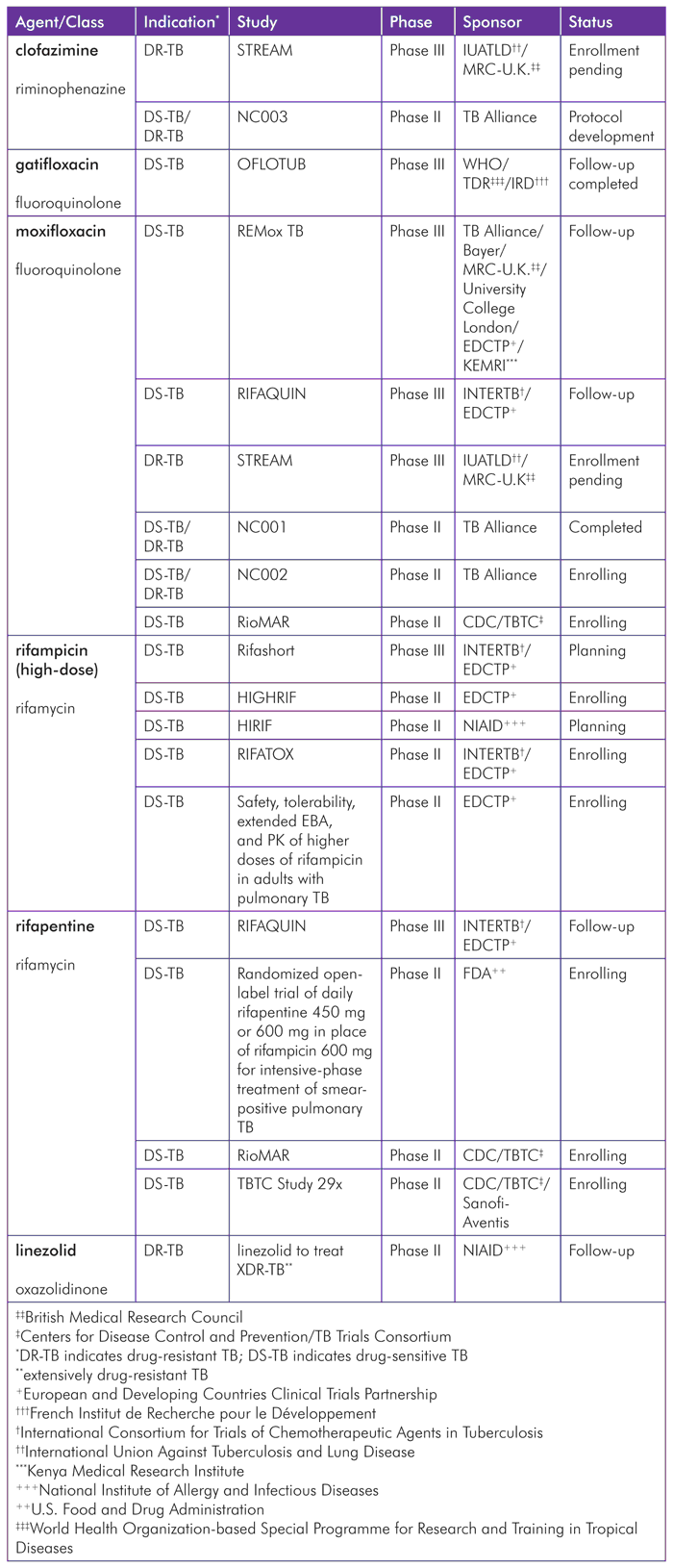
TABLE 2: Existing Drugs in Late-Stage Clinical Studies for Active Tuberculosis (TB) as of June 2012
Clofazimine
Clofazimine, a riminophenazine derivative approved for the treatment of leprosy, has long been recognized for its bactericidal activity against M. tuberculosis in mice.[42] Its inclusion in a reportedly successful nine-month standardized treatment regimen for MDR-TB, and presumed low levels of existing resistance, have contributed to increased recent interest in the drug’s potential for development as an antituberculosis agent.[43] However, its common side effect of skin discoloration (and very rare effect of accompanying depression, with two related suicides reported) and possible QT prolongation (which can lead to dangerously irregular heart rhythms) may hinder its ultimate suitability for TB treatment.[44] The results of the STREAM study and the novel combination study NC003 described later in this chapter will further illuminate the safety and efficacy of including clofazimine in new regimens to fight TB.
The International Union Against Tuberculosis and Lung Disease (IUATLD) is sponsoring the Evaluation of a Standardised Treatment Regimen of Anti-Tuberculosis Drugs for Patients with MDR-TB (STREAM) trial, funded by the U.S. Agency for International Development (USAID). It will assess a nine-month standardized treatment regimen for MDR-TB that achieved promising outcomes with a cure rate of 87.9% in a nonrandomized observational study in Bangladesh.[45] Modified after the Bangladesh regimen, which used clofazimine, ethambutol, gatifloxacin, and pyrazinamide for nine months, supplemented by prothionamide, kanamycin, and high-dose isoniazid during an intensive phase of four months, the STREAM regimen uses the same drugs, but substitutes moxifloxacin for gatifloxacin. The aim of this study is to show that this shorter treatment regimen is at least as effective as the current lengthier treatments used throughout the world to treat MDR-TB. The Clinical Trials Unit of the British MRC is conducting this trial and is expected to begin enrollment in several sites in mid-2012.[46]
Fluoroquinolones
Gatifloxacin is a fluoroquinolone, a class of broad-spectrum antibiotics. The OFLOTUB consortium’s trial replacing ethambutol with gatifloxacin aims to evaluate gatifloxacin’s potential to shorten first-line treatment to four months. The WHO-based Special Programme for Research and Training in Tropical Diseases (TDR) sponsors the OFLOTUB trial with the French Institut de Recherche pour le Développement (IRD). OFLOTUB completed the two-year posttreatment patient follow-up in April 2011, but problems in data management caused unexpected delays in data analysis. Recent progress with the database has addressed these problems, and safety and efficacy results should now be available by the end of 2012.[47]
Like gatifloxacin, moxifloxacin is a fluoroquinolone with treatment-shortening potential. The widespread use of fluoroquinolones may make existing resistance to moxifloxacin problematic; however, a recent case report exemplified the possibility of treating MDR-TB with additional resistance to fluoroquinolones (and pyrazinamide) using high-dose moxifloxacin.[48]
REMox TB is a phase III clinical trial comparing two four-month moxifloxacin-containing treatment regimens (two months of moxifloxacin/isoniazid/rifampicin/pyrazinamide plus two months of moxifloxacin/ isoniazid/rifampicin; and two months of ethambutol/moxifloxacin/rifampicin/pyrazinamide plus two months of moxifloxacin/rifampicin) for DS-TB with the standard six-month TB regimen (ethambutol/isoniazid/rifampicin/pirazinamide). REMox TB is a collaborative effort among the TB Alliance, Bayer HealthCare, University College London, University of St Andrews, the British Medical Research Council (MRC), the European and Developing Countries Clinical Trials Partnership (EDCTP), and the Kenya Medical Research Institute (KEMRI). Initiated in 2008, REMox TB has built strong community-engagement programs at several of its sites, and has helped pave the way for leveraging additional resources for TB research by its use of existing ACTG clinical trial sites.
In February 2012, the TB Alliance announced the completion of enrollment for REMox TB, after over 1,900 patients were enrolled at sites in Africa, Asia, and Latin America. The study will evaluate participants for one year following the completion of their treatment (late 2013). If data analysis shows successful trial results, TB Alliance and Bayer will seek to register moxifloxacin as part of a DS-TB treatment regimen. If approved, registration of moxifloxacin is expected in 2014.[49]
The TBTC-funded, Johns Hopkins-led RioMAR study in Brazil is also examining the role of replacing ethambutol with moxifloxacin, as well as rifamycin with rifapentine, during the intensive phase of treatment.[50] Moxifloxacin is also included in the above-described STREAM study, and in the NC001 and NC002 new combination studies described at the end of this chapter. The upcoming results of the above-described RIFAQUIN study, a pending early bactericidal activity (EBA) study by the ACTG (Study 5307) of TB regimens with and without isoniazid and moxifloxacin (isoniazid for 2 days only versus isoniazid for 2 days and moxifloxacin for 12 days, both with rifampicin/ pyrazinamide/ethambutol; versus 14 days of isoniazid/rifampicin/ethambutol/pyrazinamide), should also help clarify the potential of moxifloxacin in TB treatment.[51]
Linezolid
The U.S. National Institute of Allergy and Infectious Diseases (NIAID) sponsored a phase IIa study in South Korea, testing the side effects and effectiveness of prolonged treatment with linezolid at two different doses (in addition to other background therapy). Forty HIV-negative patients with pulmonary extensively drug-resistant TB (XDR-TB, or TB that is resistant to at least isoniazid, rifampicin, a fluoroquinolone and an injectable second-line drug) were enrolled at the National Medical Center in Seoul and the National Masan TB Hospital. Results have been submitted for publication and should be available in 2012.[52]
Rifamycins
Rifapentine, rifampicin, and rifabutin are drugs in the sterilizing drug class of rifamycins, meaning that they have excellent potential to kill all M. tuberculosis organisms present in an infection (as long as those organisms are susceptible to rifamycins).
Rifampicin is the most commonly used rifamycin in TB treatment; rifapentine and rifabutin both have longer half-lives. TBTC Study 29x, a safety study substituting rifampicin with 10, 15, and 20 mg/kg daily doses of rifapentine in the standard-of-care regimen, expects to complete enrollment by the end of the year and publish results in 2013.[53] Two open-label trials of rifapentine are also in phase II; the first, the previously described RioMAR study, which also substitutes moxifloxacin for ethambutol, is sponsored by TBTC.[54] The second is funded by the U.S. Food and Drug Administration (FDA) and conducted by the University of Cape Town Lung Institute in collaboration with the Johns Hopkins University, and is anticipated to produce results by May 2013.[55] RIFAQUIN—a phase III study being conducted by the International Consortium for Trials of Chemotherapeutic Agents in Tuberculosis (INTERTB) at St George’s, University of London, with funding from the EDCTP—is assessing whether high-dose rifapentine and moxifloxacin, when given together, can shorten first-line treatment, allow for intermittent dosing, and replace isoniazid. RIFAQUIN’s treatment phase was completed in July 2011; patients are in follow-up, and results are expected in early 2013.[56] Sanofi-Aventis is initiating a DDI study between rifapentine and ATRIPLA (a combination of the antiretrovirals efavirenz, emtricitabine, and tenofovir disoproxil fumarate) in participants with HIV who have CD4 counts greater than 400 and do not have TB; results are expected by early 2013.[57],[58]
The RIFATOX study, which examines the toxicity of 900 mg and 1200 mg daily doses of rifampicin for four months, has reached two-thirds of its target enrollment and expects results in early 2013.[59] Based on these results, a phase III study called Rifashort will look at the treatment-shortening potential of high-dose rifampicin.[60] The HIRIF study (in planning stages for initiation in late 2012), and two currently enrolling EDCTP-funded phase II studies, will help assess the pharmacokinetics and maximum tolerated dose of higher doses of rifampicin.[61],[62],[63] The ACTG is planning study A5290 to compare rifampicin- versus rifabutin-based TB treatment for patients with HIV.[64] Médecins Sans Frontières (MSF)/Epicentre is also planning RIFAVIRENZ, a DDI study between high-dose rifampicin and efavirenz.[65]
TB and injection drug use
Injection drug use is associated with many factors that place individuals at high risk for TB, including poor nutritional status, a weakened immune system, HIV and other infections, and substandard living conditions. Given the illicit nature of injection drug use in most settings, people who use injection drugs are often marginalized and have trouble accessing care. Separate silos of services for drug use, HIV, and TB make referrals to care unlikely; even worse, many drug users are imprisoned without medical services.[66] Studies in both high- and low-TB burden settings—such as Denmark, Kenya, Thailand, the United States, and Vietnam—have documented that injection drug use is associated with TB infection, disease, and death.[67],[68],[69],[70]
Operational and programmatic interventions can have a large impact on reducing TB among people who inject drugs. A recent study conducted by the Muhimbili National Hospital in Tanzania and Yale University will present its results shortly, demonstrating that active TB case finding is needed for people who inject drugs in Tanzania, to shorten the time to diagnosis, to improve individual care, and to reduce transmission of TB.[71] The Open Society Foundations’ International Harm Reduction Development program is also working to address barriers to care for people with TB who inject drugs, who face particularly harsh stigma in parts of the former Soviet Union.
In addition to programmatic improvements in access to existing diagnostic and treatment options, there is still a great need for more biomedical research to improve TB care for people who inject drugs or are on opioid substitution therapy. Rifampicin, one of the four pillars of first-line therapy for TB, reduces plasma levels of buprenorphine—a drug commonly used to treat heroin- and other opioid dependence—and induces withdrawal symptoms.[72] Rifabutin, another rifamycin, also decreases buprenorphine plasma concentrations, although it does not appear to induce withdrawal; however, rifabutin is not as commonly used as rifampicin in treating TB.[73] Further studies to optimize the dosing of buprenorphine when coadministered with rifampicin, and to examine the interactions between buprenorphine and rifapentine (another rifamycin), are needed to inform integrated TB treatment and opioid dependence services.
NOVEL COMPOUNDS TO TREAT ACTIVE TB DISEASE
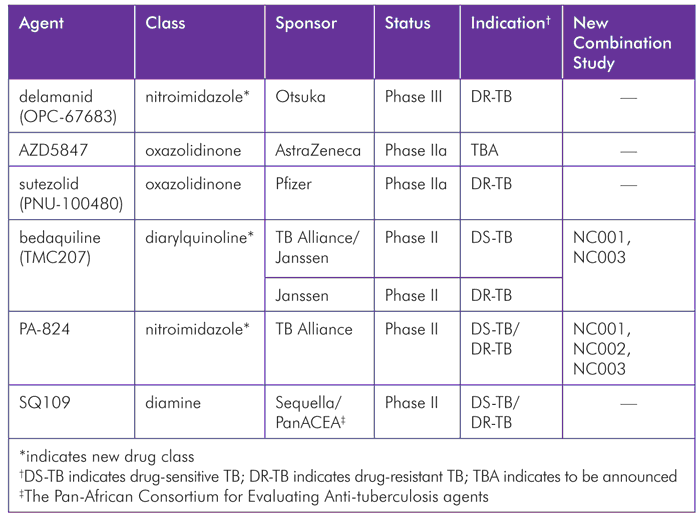
TABLE 3. Novel and Second-Generation Compounds in Late-Stage Clinical Studies for Active Tuberculosis (TB) as of June 2012
AZD5847
AZD5847 is an oxazolidinone in development by AstraZeneca for the treatment of pulmonary tuberculosis. AstraZeneca’s development of AZD5847 helped make it the third-largest private funder of TB R&D in 2011.[74] Oral AZD5847 has been investigated in two phase I studies—a single ascending dose, and a multiple ascending dose where the drug was administered up to 1,200 mg twice a day for a period of 14 consecutive days. In the former, the pharmacokinetics of AZD5847 were also compared when administered fasting versus fed: administration with food significantly increased its bioavailability and improved gastrointestinal tolerability.
Generally, the compound was well tolerated, and no major safety concerns were identified in either study. There were no clinically relevant treatment-related changes or trends in any laboratory variables, vital signs, or electrocardiograms. In the multiple ascending-dose study, the most common adverse effects were nonserious gastrointestinal disorders; reversible and dose-related changes in white blood cells; and mildly increased reticulocyte counts at follow-up. Changes in reticulocyte counts were not accompanied by changes in hemoglobin or hematocrit measurements.[75],[76],[77] A phase IIa study is proposed to begin in the fall of 2012 in patients with DS pulmonary tuberculosis. AstraZeneca has not yet determined if the compound will ultimately be developed for DS-TB or DR-TB.[78]
Bedaquiline (TMC207)
Bedaquiline (also known as TMC207) is the first compound from a new class of drugs called diarylquinolines. Bedaquiline is being developed for DR-TB by Janssen Infectious Diseases BVBA (a subsidiary of Johnson & Johnson formerly known as Tibotec), and for DS-TB by Janssen and the TB Alliance. As noted above, in early July 2012, Janssen filed its NDA with the U.S. FDA for accelerated approval of bedaquline for treatment of DR-TB.[4]
In late 2011, Janssen presented 24-week data from an open-label trial of bedaquiline in adults with smear-positive, confirmed MDR-TB or XDR-TB, including patients with HIV. The data indicated that adding bedaquiline to an individualized MDR-TB regimen was safe and well tolerated and resulted in an overall 81% culture conversion rate at week 24, with median times to culture conversion of 8 weeks for patients with MDR-TB, 12 weeks for patients with pre-XDR-TB, and 24 weeks for patients with XDR-TB. Responder rates were higher for patients with no cavitations (holes in the lungs caused by extensive cell death), patients with a lower extent of resistance, and patients on three or more potentially active drugs in their background regimen. Patients in the trial are being followed while they complete their background regimen.[79]
A recent publication of the two-year follow-up results for a randomized study of 47 patients with pulmonary MDR-TB treated with either bedaquiline or placebo added to the first eight weeks of a background regimen showed that bedaquiline significantly reduced the time to culture conversion over 24 weeks, and was comparable to placebo in terms of adverse events (with the exception of nausea, which bedaquiline caused in more patients). Additionally, though the numbers were small, only one patient receiving bedaquiline acquired resistance to companion drugs (excluding ethambutol and ethionamide) versus five patients receiving placebo.[80]Though the number of study participants involved was small, and the difference in acquired resistance not significant, there is now evidence that the addition of bedaquiline to current MDR-TB regimens may have the potential to reduce resistance.
Janssen now plans to start a phase III trial of 600 subjects with sputum smear–positive pulmonary MDR- or pre-XDR-TB (confirmed by rapid diagnostic test). Participants in the first arm will receive 9 months of bedaquiline and a background regimen. Those in the control arm will receive placebo and the background regimen. Participants in a third rollover arm, which will capture the failures from the first two arms, will receive an individualized salvage regimen. The primary endpoint will be relapse-free cure at 15 months for those in the first two arms. The final analysis will look at relapse-free cure at 21 months.[81]
Janssen is also taking into consideration TB/HIV-coinfection and pediatric DR-TB in its development plans. The pediatric investigational plan that will guide future clinical studies of bedaquiline in children to establish safe and effective dosing based on age and development has been approved by the EMA and has been shared with the FDA.[82]
The IMPAACT network is currently finalizing the protocol for Study 1108, a pharmacokinetic and safety study of bedaquiline in children with MDR-TB. This study of different age cohorts will begin by placing the oldest children (12–18 years) on an adult formulation of bedaquiline. All younger cohorts (6–12 years, 2–6 years, 6 months–2 years, 0–6 months) will be placed on a pediatric formulation currently in development by Janssen, sequentially from oldest to youngest, once adequate data from the preceding cohort are available. Enrollment is anticipated to start in the first quarter of 2013. The study plans to first enroll HIV-uninfected children in each age cohort, then enroll similar numbers of HIV-infected children, all with proven or presumed MDR-TB. This study is an excellent example of an appropriate pediatric study design that also takes into account TB/HIV coinfection, and of a public-private partnership.[83]
Janssen is conducting DDI studies with ARVs known to inhibit cytochrome P450, a group of enzymes that metabolize bedaquiline. Coadministration with the boosted protease inhibitor lopinavir/ritonavir increased exposure to bedaquiline by approximately 20%, and a trial with nevirapine (NVP) indicated that steady-state NVP did not influence exposure to bedaquiline or its metabolite, and single-dose bedaquiline did not influence pre-dose NVP concentrations.[84] An ACTG-led DDI study of bedaquiline and efavirenz (EVF) similarly showed that single-dose bedaquiline was well tolerated alone and with steady-state EFV, and that changes in bedaquiline concentrations when given with EFV are unlikely to be clinically significant. The DDI results with repeated dosing of bedaquiline have not yet been studied. Further data will be presented by the ACTG at the International Workshop on Clinical Pharmacology of Tuberculosis Drugs in September 2012.
The NIAID Division of Microbiology and Infectious Diseases (DMID) has just completed enrollment of a phase I study examining drug-drug interactions between bedaquiline and rifampin and rifabutin. Final results of this study will be available in late 2012.[85]
Delamanid (OPC-67683)
Delamanid, a nitroimidazole formerly known as OPC-67683, is a novel compound being studied for the treatment of MDR-TB. Delamanid is in a new class of compounds that inhibit mycolic-acid biosynthesis with specificity to mycobacteria, especially M. tuberculosis.[86]The specificity of nitroimidazoles’ action to the TB bacterium prevents their use for other indications, meaning that widespread resistance generated outside of TB control programs would be unlikely.
Otsuka just published the results of a phase IIb study of two different doses of delamanid plus optimized background regimen (OBR) versus placebo plus OBR in 481 volunteers with confirmed MDR-TB. Of patients receiving 100 mg or 200 mg of delamanid plus OBR, 45.4% and 41.9%, respectively, had sputum culture conversion at two months, as compared with 29.6% of patients receiving OBR and placebo. QT prolongation was more common among those receiving delamanid, although no clinical events due to QT prolongation were observed, and in general, most adverse events were mild to moderate and were distributed evenly across study arms.[87]Thus, delamanid appears efficacious and safe for use as part of an MDR-TB regimen.
As part of the phase IIb trial, a long-term, open-label surveillance of patients with MDR-TB who have been treated with delamanid and OBR is also under way to extend the efficacy and safety observations from the trial, and to further document the durability of response. The results of these studies, and of DDI studies with ARVs, will be published soon in a peer-reviewed journal.[88]
Otsuka recently initiated an international phase III clinical study with delamanid. The randomized controlled trial includes six months of treatment with delamanid as part of a full course of treatment with OBR, and includes HIV-coinfected MDR-TB patients.[89]
Otsuka filed in late 2011 for European Medicines Agency (EMA) approval for delamanid for treatment of DR-TB; a decision is anticipated in 2013. This makes delamanid the first new anti-TB drug, and nitroimidazoles the first new class of anti-TB drugs, submitted for approval by a stringent regulatory authority in the past four decades. Otsuka’s investments to develop delamanid make it the leading funder of TB drug R&D, and the leading private-sector funder of TB R&D overall.[90]
Pediatric TB
Children have long been neglected in the fight against TB, despite making up 15–20% of the global tuberculosis burden.[91] Difficulties in diagnosing TB in children, and the notion that children do not transmit TB, have contributed to this neglect. In particular, few children with DR-TB receive appropriate diagnosis and treatment, despite evidence that even children with MDR-TB can be treated successfully.[92],[93] Fortunately, increased attention to fighting pediatric TB is building.
The general principles of treating adults and children are the same.[94] However, children and adults metabolize drugs differently; therefore, doses for children cannot be determined simply by scaling down the adult dose per kilogram.[95] The 2010 WHO guidelines accounted for these differences and updated the recommended dosages of isoniazid, ethambutol, pyrazinamide, and rifampicin. However, the pediatric formulations available on the market today are not tailored to deliver the new dosages, and complex interim dosing guidelines using the current unsuitable fixed-dose combination (FDC) have hindered the implementation of these new recommendations. Treatment providers including MSF have been lobbying the WHO to release recommendations for the composition of a new FDC for pediatric first-line TB treatment that corresponds with these new dosing guidelines. Once the new FDC formulations are on the WHO prequalification expression-of-interest list for drug manufacturers, there will be a need to engage manufacturers to start developing these formulations as quickly as possible. This will facilitate the appropriate and prompt dosing and treatment of DS-TB in children.
To help address the issue of dosing in children, Lucane Pharma recently developed a child-friendly dosing spoon. This tool is validated for the MDR-TB product Paser (p-aminosalicylic acid), a gastroresistant (meaning it is absorbed in the duodenum rather than the stomach, where it could cause damage) second-line drug whose conventional packaging consists of granules in a sachet. The dosing spoon helps care providers measure smaller doses of PAS accurately. As of February 2012, the spoon is being used in Uzbekistan and Tajikistan by MSF, as well as in South Africa by the Desmond Tutu TB Centre in Stellenbosch, and in France. Lucane is working on a similar preparation for isoniazid, and is open to developing similar pediatric-friendly formulations and dosing tools for other drugs useful in the treatment of childhood DS- and DR-TB.[96]
Pediatric DR-TB is also beginning to receive other much-needed attention, thanks in part to the efforts of the Sentinel Project on Pediatric Drug-Resistant Tuberculosis, a group of experts and stakeholders in childhood DR-TB.[97] To facilitate both research and the clinical management of pediatric DR-TB, the group has established standardized definitions for measures of exposure, resistance, site and severity of disease, adverse events, and treatment outcomes.[98] The group is simultaneously publishing a handbook to serve as a practical management tool for pediatric DR-TB to help guide practitioners in the field.[99] Both the definitions and the handbook should be published shortly.[100],[101]
PA-824
PA-824, like delamanid, is from a new drug class, the nitroimidazoles. In phase II development by the TB Alliance, PA-824 recently was shown to be safe, well tolerated, and efficacious at doses of 100–200 mg daily in a dose-ranging study among drug-sensitive, sputum smear–positive, adult pulmonary TB patients. The TB Alliance will evaluate PA-824 as a component of novel anti-TB regimens for both DS-TB and DR-TB moving forward.[102] These PA-824-containing novel regimens are being tested in the combination studies described further in the next section of this chapter.
The NIAID DMID and the TB Alliance are cosponsoring a phase I thorough QT (TQT) study to evaluate any effects PA-824 will have on cardiac conduction (the rate at which the heart conducts electrical impulses). The clinical trial will also study whether PA-824 and moxifloxacin had additive or synergistic effects on the QT interval. This study will start enrolling in Q4 2012.[103]
The ACTG has opened a phase I safety, tolerability, and pharmacokinetic interaction study of PA-824 and two common antiretrovirals (ARVs).[104] This study, also called A5306, will look at whether PA-824 is safe to use with lopinavir/ritonavir (a boosted protease inhibitor) and efavirenz (a non-nucleoside reverse transcriptase inhibitor) as well as with rifampicin.[105],[106] Given the prevalence of TB/HIV coinfection, these DDI studies between new potential anti-TB agents and commonly used ARVs are essential.
In late 2012, the TB Alliance plans to file an investigational new drug (IND) application with the FDA, and to start a phase I program for its backup nitroimidazole, the new drug candidate TBA354, which is currently in preclinical development.[107]
SQ109
SQ109, a second-generation ethylene diamine antibiotic, is the lead compound from Sequella. In a phase IIa early bactericidal study, with collaborators from the Pan African Consortium for Evaluating Antituberculosis Agents (PanACEA), Sequella was found to be safe and well tolerated and will be evaluated in a multiple-arm, multiple-stage, phase II study in DS-TB expected to start in 2012. Additional studies in DS- and DR-TB are planned for late 2012/early 2013 with the ACTG. In parallel, Sequella and the Maxwell Biotech Venture Fund plan a late-2012 phase III study of SQ109 for DR-TB in Russia, Armenia, Azerbaijan, Belarus, Kazakhstan, Kyrgyzstan, Moldova, Tajikistan, Uzbekistan, and possibly Turkmenistan and Ukraine.[108]
Sutezolid (PNU-100480)
Sutezolid, or PNU-100480, is a new oxazolidinone—the same class of drugs as linezolid. Sutezolid appears to have more potent antituberculosis activity in vitro, in ex vivo whole blood cultures, and in a murine (mouse) model.[109],[110],[111],[112],[113] A whole-blood study also predicted that sutezolid and two drugs described above—SQ109 and bedaquiline—would be additive, and deserve testing as part of a novel regimen.[114] Pfizer recently completed a phase IIa, open-label, early bactericidal activity and whole-blood activity study. This study of adults with pulmonary DS-TB compared two experimental arms—one with sutezolid twice daily at 600 mg, the other with sutezolid once daily at 1,200 mg—with Rifafour. Results should be available soon.[115]
HIV/TB integration
The WHO’s recently updated policy on collaborative TB/HIV activities highlights important steps to integrating TB and HIV service delivery, including increasing case finding; initiating IPT and antiretroviral therapy in individuals with HIV; providing HIV testing and prevention interventions for patients with TB; and ensuring TB infection control in health care facilities.[116] On the research side, DDI studies between commonly used ARVs and TB drugs (both existing and new) are necessary to ensure that coadministration is safe.
In most cases, clearly, it is essential to initiate antiretroviral therapy (ART) as soon as is practicable after starting TB treatment in persons coinfected with HIV; the one exception appears to be in cases of tuberculous meningitis. The Cambodian Early versus Late Introduction of Antiretroviral Drugs (CAMELIA) study showed that initiating ART two weeks versus eight weeks after initiating TB treatment significantly improved survival among HIV-infected adults with CD4 counts below 200 (it is important to note that the average baseline CD4 cell count for this trial was 25).[117] Two other simultaneously published randomized trials, ACTG Study 5221 (START) and the Starting Antiretroviral Therapy at Three Points in Tuberculosis (SAPIT) study showed that earlier ART initiation (within two to four weeks of beginning TB treatment) in people with HIV and CD4 counts below 50 increased survival, but that in individuals with higher CD4 counts, deferral of ART initiation to the continuation phase of TB therapy (two to three months after initiation) may reduce the risk of immune reconstitution inflammatory syndrome and other adverse events without increasing the risk of AIDS or death.[118],[119] The average baseline CD4 cell count was 77 for START, and 150 for SAPIT. These study participants clearly did not have HIV disease as advanced as did those in the CAMELIA trial, which may explain the apparent difference in results. A planned meta-analysis of these three trials should help better define criteria and timing for initiation of ART after starting TB treatment.[120]
However, in a randomized, double-blind, placebo-controlled trial of immediate ART versus deferred ART (study entry or two months later) in patients with HIV-associated tuberculous meningitis (who are excluded from the usual TB treatment trials because they need special care due to central nervous system inflammation and higher rates of morbidity and mortality), no difference was found in mortality or the time to new AIDS events or death. Grade 4 adverse events occurred significantly more frequently in the immediate-ART arm. These results support delayed initiation of ART in HIV-associated tuberculous meningitis.[121]
ACTG study A5274, the REMEMBER Study, should shed light on a different aspect of the “when to start” issue by looking at whether full four-drug treatment for active TB should be started only after active TB infection is found, or whether people with HIV and CD4 counts below 50 do better on “empiric” four-drug TB treatment even if they have not been diagnosed with active TB.[122] A similar study called PROMPT is being conducted in Africa with funding from the EDCPT.[123]
NOVEL COMBINATIONS TO TREAT ACTIVE TB DISEASE
TB treatment requires the delivery of multiple drugs in combination so as to prevent the development of resistance. The traditional drug development paradigm involves substituting a drug in the standard-of-care regimen with one novel compound at a time. In this traditional paradigm, producing a novel regimen can take at least 20 years (the British MRC took 38 years to move from streptomycin monotherapy in 1948 to the standard of care of two months of isoniaizid/rifampicin/pyrazinamide/ethambutol [HRZE] followed by four months of isoniazid/rifampicin in 1986).[124] While early research on individual new drugs is critical to determining safety profiles, efficacy, and dosing, late-stage studies combining multiple novel compounds have the potential to rapidly catalyze regimen change and provide the millions of people with TB access to better care.
To encourage this paradigm shift and shorten the TB drug development timeline, the Critical Path to TB Drug Regimens (CPTR) initiative was founded in 2010 by the Bill & Melinda Gates Foundation, the Critical Path Institute, and the TB Alliance.[125] The FDA has also been instrumental, issuing a guidance in 2010 to facilitate combination studies, awarding support for clinical trials, and in 2012 proposing to lower the risk classification of nucleic acid–based tests for TB to encourage the development of new rapid diagnostic tests for TB. The TB Alliance has made progress on three early-stage (two-week EBA and two-month sputum serial colony count [SSCC]) combination trials, as described below.[126] These combination trials represent a new, potentially unified pathway for DS- and DR-TB drug development, in which drugs in combination are indicated for the treatment of tuberculosis caused by strains sensitive to each drug.[127] That is, patients are treated with drugs to which their organism is sensitive, rather than with combinations against which it is thought they are not resistant; however, this development will require drug-susceptibility testing that takes less than one day—something that now exists only for isoniazid, rifampicin, fluouroquinolones, and injectables).[128]
NC001
The TB Alliance recently completed NC001, the first TB clinical trial to evaluate multiple unapproved new TB drug candidates in combination. This two-week, phase II EBA study tested the three-drug regimen PA-824, moxifloxacin, and pyrazinamide (PaMZ). The PaMZ regimen performed significantly better than the standard of care (HRZE).
The study also tested additional two-drug combinations of PA-824, moxifloxacin, and pyrazinamide and bedaquiline to evaluate their potential as “building blocks” of future regimens. Validating what had been seen in mouse models, pyrazinamide and bedaquiline were synergistic, pyrazinamide and PA-824 had an additive effect, and PA-824 and bedaquiline did not have an additive effect.
The study was also important for helping open up a promising new regulatory pathway for new combination trials. In addition, it demonstrated that EBA studies can distinguish between treatments, not just between doses of the same treatment. NC001 also showed that measuring colony-forming units (which involves comparing the number of remaining viable bacterial cells that can grow into colonies after the experimental and control treatment) and time to positivity (TTP, which measures how long a cultured sputum sample takes to read as positive after therapy, with more effective treatment leaving fewer live bacterial cells and therefore having a longer TTP) gave similar results, helping to validate TTP as a biomarker for treatment response.[129],[130]
NC002
Based on the results of NC001, the TB Alliance is evaluating PaMZ for both DS- and DR-TB in NC002, a two-month serial sputum-colony-counting (SSCC) study; SSCC measures the fall in the number of viable counts of M. tuberculosis in samples collected under standardized conditions on multiple occasions before and after initiating therapy.[131] The study is slated to take place at eight sites in South Africa, Tanzania, and Brazil, and will advance global capacity for TB trials along with the new innovative approach to TB drug development.[132] This study started enrolling patients in the first quarter of 2012, with results expected in the summer of 2013.[133]
NC003
The TB Alliance is also planning study NC003, which will evaluate the EBA, safety, tolerability, and pharmacokinetics of two weeks of once-daily oral dosing of clofazimine alone, pyrazinamide alone, and various combinations of these drugs with PA-824 and bedaquiline, in comparison with standard first-line TB treatment. NC003 will enroll 105 newly diagnosed adults with smear-positive, drug-sensitive pulmonary tuberculosis. The study is expected to begin enrolling patients in late 2012, with results expected in the summer of 2013.[134]
Preapproval access to compounds
People with XDR-TB and pre-XDR-TB have drastically limited treatment options. Those that exist are effective for only some patients and have major toxicity issues. Moreover, they are often unavailable in communities where they are needed. People with XDR-TB and pre-XDR-TB can’t afford to wait the several years it will take for new compounds in development to become approved and available.
The compassionate use of these compounds could potentially provide a lifesaving option to patients otherwise without hope. Compassionate use refers to preapproval provision of compounds to patients meeting strict criteria specified by the local government and the drug sponsor. Expanded access, meaning the extension of a clinical trial to select patients in need who would not normally meet study inclusion criteria, is also an option where compassionate use is not permitted. In both pre- and postapproval instances, new drugs must be used cautiously and in combination with other effective background therapy; this will ensure that further resistance does not develop, and preserve the new compound as an effective treatment option for as many MDR-TB patients as possible.
Based on its HIV experience, Janssen has taken laudable steps to provide the compassionate use of bedaquiline in Europe, the Americas, Africa, and Asia.[135] Otsuka is in the process of developing approaches and models of compassionate use and expanded access for delamanid.[136] The TB Alliance is also proposing a collaborative investigational “rescue” study of a combination of at least three new drugs in development from novel classes (meaning they will not face preexisting resistance) in patients with XDR-TB. Potential collaborators include Otsuka, Janssen, Pfizer, and possibly Sequella. Treatment with three new drugs, instead of just one, is expected to ensure both adequate treatment and prevention of resistance development. The goal is to provide real help to patients with treatment-resistant forms of TB as soon as possible, while simultaneously gathering intensive data on outcomes with long-term follow-up. This proposed global study of combinations of new chemical entities at select centers of care is expected to have only a marginal incremental cost compared with traditional, individual compassionate use and expanded access programs.[137]
With drugs in late-stage clinical development, sufficient efficacy, safety, and pharmacokinetic data exist or will soon exist to justify their compassionate use. However, there is unacceptable resistance within the TB establishment to allowing compassionate use. Regulators and controllers in many countries are reluctant to allow access to well-characterized compounds in development, opting instead to continue putting people on toxic fourth- and fifth-line drugs, which often have even less available evidence of safety and efficacy than do investigational drugs. Denying people with XDR-TB and pre-XDR-TB the chance to benefit from new drugs is, in many cases, a death sentence. Providing rational, expeditious preapproval access to new compounds—ideally in combination—is essential.
Recommendations
Pediatric and Maternal TB
Integrating TB screening and treatment for women and children into HIV and other health-service programs is a simple and efficient way to involve these often-neglected populations. In particular, IPT for children and pregnant women is often unavailable in many countries, and could be facilitated with a simple tool to improve IPT management in child contacts.[138],[139] Research also needs to involve women, including pregnant and breastfeeding women, earlier and more often. Pediatric investigational plans for drug development need to involve children—including very young children—as early as safety permits. The timely development of pediatric-friendly formulations and FDCs will facilitate the earlier inclusion of children in research and the appropriate administration of treatment in programmatic settings.
Regulatory Requirements
Stringent regulatory authorities should consider logical and innovative clinical trial study designs such as symptomatic and microbiological endpoints to make drug development more efficient. Regulatory authorities in countries with high TB burdens need to streamline their clinical trial– and drug approval processes to allow their constituents to access the life-saving benefits of crucial research and new drugs, once developed.
TB Elimination
TB research and programmatic efforts have led to major advances in the fight against tuberculosis. Combination studies are revolutionizing the TB drug development paradigm, and two drug candidates from novel classes are poised for registration. Operational research has demonstrated the role of case finding, preventive therapy, and TB/HIV service integration in reducing TB. A recent study showed that there was an association between increasing investments in national treatment programs and improved performance in reducing the TB burden in the 22 high-burden countries.[140] However, a perilous funding climate threatens these advances. Sustained—and indeed increased—political will to fight TB is necessary. The TB community—including researchers, sponsors, donors, multilateral policy makers, regulators, implementers, TB-infected individuals, and TB-affected community advocates—needs to continue building momentum to not just control, but finally eliminate TB.
Postscript
October 2012 Update
BEDAQUILINE: In June 2012, Janssen filed its new drug application with the U.S. Food and Drug Administration (FDA) for accelerated approval of bedaquiline for the treatment of MDR-TB. The FDA will hold a public hearing on bedaquiline on November 28, 2012, and is scheduled to respond to the bedaquiline application by December 29, 2012. Janssen also filed with the European Medicines Agency (EMA) in August 2012.
February 2013 Update
BEDAQUILINE: In December 2012, the U.S. Food and Drug Administration (FDA) granted bedaquiline accelerated approval for the treatment of adults with MDR-TB. Bedaquiline (also known by its trade name, Sirturo) is the first truly novel drug to fight TB to be approved in four decades. Janssen, the drug’s sponsor, is starting to file for approval in other countries, and the World Health Organization plans to release guidelines in the first half of 2013 for the drug’s use.
While access to bedaquiline is critical given the paucity of effective and tolerable treatment options for people with drug-resistant TB, further research is still necessary. Janssen must carry out a phase III study to demonstrate bedaquiline’s safety. Additional studies are also essential to determine bedaquiline’s suitability for use in children, in people who use alcohol and drugs, and along with antiretrovirals and other new TB drugs.
