February 2013 Update
On Feburary 4, 2013, results from the phase IIb trial of the MVA85A vaccine in infants in South Africa (given as a booster following BCG immunization) were published in the Lancet. The vaccine proved safe but, disappointingly, failed to show signifcant efficacy. However, as the researchers note, this was the first infant efficacy trial of a new TB vaccine since BCG was last assessed as part of the Chengalpattu trial in 1968; as such, it is a major milestone for the field, and analyses of the results, including assessments of the immune responses induced by the vaccine, will make a vital contribution to advancing the development of new vaccine candidates. Results from trials in adults are pending, and it remains uncertain if the lack of efficacy in infants will be mirrored in the adult setting.
Additional resources:
The Lancet podcast: Helen McShane from the University of Oxford discusses results from a phase IIb tuberculosis trial
Trial sponsors press release: Data from Historic Phase IIb Clinical Trial for Tuberculosis Vaccine Candidate MVA85A Published in the Lancet
Action Global Health Advocacy Partnership: TB Vaccine Trial Results: A Launching Pad for Further Research
July 2012
By Richard Jefferys
The human immune system and Mycobacterium tuberculosis (MTB), the causative agent of tuberculosis (TB), are ancient adversaries. MTB DNA has been detected in skeletal and mummified samples dating back to thousands of years BCE.[1] As it stands today, the two sides have reached an imperfect, partial détente. The vast majority (~90%) of infected individuals resist disease through immunologic mechanisms that are only partly understood, but a significant proportion go on to develop active TB disease. The risk of active disease is greatly increased among those with immune deficiencies; while the lifetime risk is normally around 10%,[2],[3] it can exceed 10% per year for HIV-positive people.[4]
The goal of vaccination is to improve the immune response to TB and reduce the incidence of active disease, either by enhancing control of the infection or preventing it entirely. Early last century, it was hoped that an attenuated version of a related organism, Mycobacterium bovis bacille Calmette–Guérin (BCG), would be effective. But while BCG remains the only licensed TB vaccine, it has turned out to offer highly variable and generally inadequate protection against the most common pulmonary form of the disease. BCG remains in use in many parts of the world due to its ability to protect against disseminated forms of TB during childhood, however it is no longer recommended for HIV-positive children due to the risk of disease from the vaccine itself.[5]
The partial efficacy of BCG is evidence that a TB vaccine is possible, but there is an urgent need to develop superior approaches. It has been estimated that even a 60% effective candidate could reduce TB incidence approximately 80% by 2050.[6] Yet society has been slow to recognize this urgency—as of the early 1990s, the pipeline of new TB vaccine candidates was completely empty. The situation has improved considerably over the past two decades, with much of the initial impetus coming from a workshop entitled “Blueprint for Tuberculosis Vaccine Development” chaired by scientist Barry Bloom in 1998.[7]
There are currently 12 new TB vaccine candidates in human trials, and the work begun at the 1998 workshop has been updated with the release earlier this year of “Tuberculosis Vaccines: A Strategic Blueprint for the Next Decade,” a collection of papers that were initiated by discussions at the 2nd Global Forum on TB Vaccines in Tallinn, Estonia, in 2010 and published in the journal Tuberculosis in March 2012.[8] The process that led to the updated blueprint was coordinated by the World Health Organization’s (WHO) Stop TB Partnership’s Working Group on New Vaccines, and supported by the WHO, the Bill & Melinda Gates Foundation, Aeras, the TuBerculosis Vaccine Initiative (TBVI), and the U.S. National Institutes of Health (NIH). The stakeholders involved are now meeting on a more regular basis, with the 3rd Global Forum on TB Vaccines scheduled to take place March 24–27, 2013, in Cape Town, South Africa. Although still underresourced, the field has benefited from an influx of over US$600 million in funding in the period 2005–2010[9].
Over the past year, two new TB vaccine candidates have entered clinical trials, and there have been other signs of progress. These include:
- Discussions between the Aeras and the mining conglomerate Anglo American to explore the possibility of TB vaccine trials in the latter company’s South African mines, where workers face the world’s highest risk of the disease;[10]
- A collaboration between the National Institute of Allergy and Infectious Diseases (NIAID), Aeras, and the company Crucell N.V. that will leverage NIAID’s clinical trial infrastructure to provide additional sites for a pediatric study of the AERAS-402/Crucell Ad35 candidate TB vaccine as a booster immunization to BCG;[11] and
- A strengthened collaboration between Aeras and the European-based TuBerculosis Vaccine Initiative (TBVI) that will work to implement the recommendations of the new strategic blueprint for TB vaccines.[12]
The Vaccine Clinical Pipeline
There are two broad categories of vaccines under study: replacements for BCG that aim to be safer and more effective, and several approaches to boost immune responses to selected TB antigens. As yet there are no clear correlates of immunity to TB—a problem similar to that faced by HIV vaccine developers—so candidates face a long and costly journey through the pipeline, culminating in large-scale trials to prove efficacy. It has been proposed that it may be possible to test the ability of vaccine-induced immune responses to control a BCG challenge in humans, which could potentially help identify correlates of protection, but this model is still in the early stages of development.[13] Current knowledge regarding protective immune responses to TB, and their application to vaccine development, are the subject of an excellent and comprehensive recent review by Tom Ottenhoff and Stefan Kaufmann in the open access journal PLoS Pathogens.[14]
TABLE 1. TB Vaccine Candidates in Clinical Trials (as of July 2012)
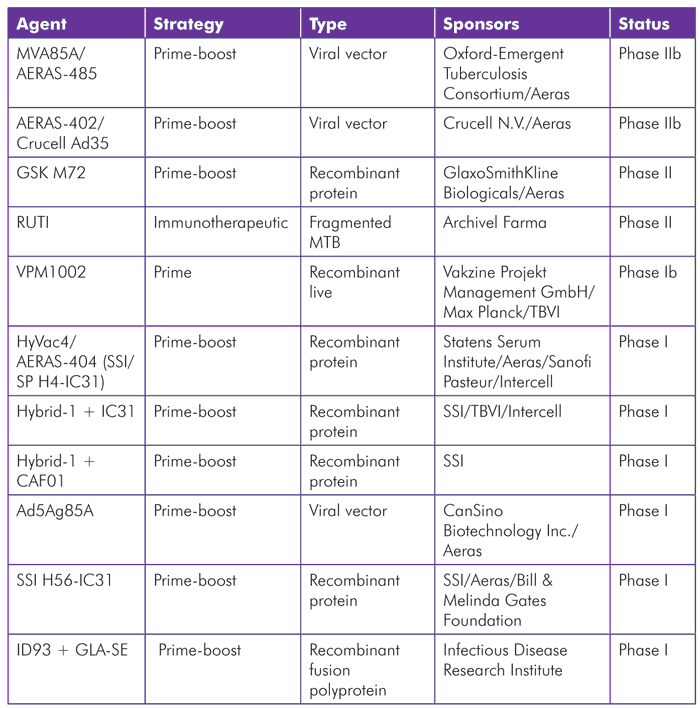
MVA85a/AERAS-485
MVA85A/AERAS-485—a recombinant attenuated version of the vaccinia virus (cowpox) combined with TB antigen 85A—is among the most clinically advanced TB vaccines. It was developed at Oxford University and is being evaluated as a booster of preexisting immune responses to antigen 85A, which are present in most people either as a result of BCG vaccination or natural exposure to TB. Phase I and II safety studies indicate that the vaccine has an acceptable safety profile; the most common side effects include local site of injection reactions and flu-like symptoms. Published immunogenicity results show induction of CD4 T-cell responses, which have generally been long-lasting and show a polyfunctional profile (meaning an ability to secrete multiple cytokines).[15] Findings from studies in HIV-positive individuals are similar, but the CD4 T-cell responses have tended to be of lower magnitude and less durable. HIV status does not affect the safety of the vaccine, and—in collaboration with the Vaccine Research Center at NIH—the researchers have demonstrated that antigen 85A-specific CD4 T cells induced by the vaccine do not become preferentially infected by HIV.[16]
Aeras has partnered with the Oxford-Emergent Tuberculosis Consortium Ltd. (OETC) on a phase IIb efficacy trial of this candidate in infants that completed enrollment in April 2011. A second phase IIb efficacy trial in HIV-positive adults, funded by the European and Developing Countries Clinical Trials Partnership (EDCTP), is now under way. The OETC, a joint venture between the University of Oxford and Emergent BioSolutions Inc., has the rights to fully commercialize the vaccine, and Aeras will have the rights to distribute the vaccine to resource-limited populations for humanitarian purposes.
AERAS-402/Crucell Ad35
AERAS-402/Crucell Ad35 comprises a replication-deficient adenovirus serotype 35 (Ad35) that serves as a viral vector—a virus modified to deliver TB genetic material—for DNA-expressing TB antigens 85A, 85B, and 10.4. Aeras and Crucell N.V., a Dutch biopharmaceutical company that focuses on developing adenovirus-based vaccines for infectious diseases, are developing this vaccine candidate. Adenoviruses are unusually potent inducers of CD8 T-cell responses, which are considered an important component of immunity to TB and many other infections.
When given in adults after priming with BCG, AERAS-402/Crucell Ad35 has been shown to induce polyfunctional CD4 T cells and strong CD8 T-cell responses that were fiftyfold higher than those detectable pre-boost (the highest magnitude CD8 T-cell responses seen with any candidate to date).[17] A phase IIb proof-of-concept clinical trial in HIV-negative infants ages 16–26 weeks is ongoing. The study includes an initial dose-finding period, followed by a safety and efficacy phase that will recruit over 4,000 infants (the recently announced collaboration with NIAID is providing additional sites for this trial). A phase II trial evaluating the safety and immunogenicity of AERAS-402/Crucell Ad35 in HIV-infected, BCG-vaccinated adults with greater than 350 CD4 T cells was initiated in 2009, but is currently on hold pending additional funding.
GSK M72
GlaxoSmithKline (GSK) is working with Aeras to conduct phase II studies of GSK M72, a recombinant protein vaccine combined with a proprietary adjuvant, AS01. Early results show that the vaccine is well tolerated and induces robust polyfunctional CD4 T-cell responses against the M72 antigen that have persisted for at least three years, but no CD8 T-cell responses. No serious adverse events have occurred; the main side effects are transient local injection-site reactions.[18] A phase II study assessing the safety and immunogenicity in HIV-positive adults with or without ART in TB endemic areas has closed to recruitment but continues to follow participants. Another ongoing phase II trial in Taiwan is assessing the impact of the vaccine in HIV-negative individuals who have received, or are currently receiving, treatment for active TB.
RUTI
RUTI is a killed TB vaccine originally discovered at Institut Germans Trias i Pujol and now being developed by the biotech company Archivel Farma. The vaccine is being evaluated for its potential to accelerate the treatment of latent TB infection in combination with isoniazid. A phase II study that compared three different doses of RUTI given after one month of isoniazid in HIV-positive and HIV-negative adults has been completed. The vaccine was well tolerated, with the most common adverse events being mild injection-site reactions. No effects on CD4 T cell counts or viral load were observed among HIV-positive participants. The vaccine induced long-term memory T-cell responses to multiple TB antigens.[19] Based on these results, a single injection of a 25 μg dose has been selected for evaluation in a proposed phase III efficacy trial to evaluate whether vaccination after six months of isoniazid can reduce the incidence of active TB among HIV-positive individuals with latent TB infection.
HyVac4/AERAS-404 (SSI/SP H4-IC31), Hybrid 1 + IC31, Hybrid 1 + CAF01, and SSI H56-IC31
The Statens Serum Institut (SSI) is a Danish research institution developing several TB vaccine candidates. The SSI strategy involves the use of protein subunits comprising different TB antigens combined into fusion molecules. These vaccines are currently being tested in combination with different adjuvants.
HyVac4/AERAS-404, also referred to as SSI/SP H4-IC31, uses SSI’s H4 antigen (a fusion protein of 85B and 10.4) combined with Intercell’s IC31 adjuvant. SSI is partnering with Aeras, TBVI, Intercell, and Sanofi Pasteur to develop this construct. Aeras is currently conducting a phase I trial in healthy adults.
Hybrid 1 contains the TB antigens 85B and ESAT6, and has been studied in combination with either IC31 or CAF01 adjuvants. With IC31, it has been shown to induce memory T-cell responses that were maintained over 2.5 years of follow-up in BCG-naive volunteers,[20] and it also enhanced TB-specific immune responses in a study including individuals with prior BCG vaccination or TB infection.[21] A phase IIa trial of Hybrid 1 with IC31 is being planned.
SSI has published promising preclinical data on its newest candidate that includes a novel latency-associated TB antigen, Rv2660c, along with Ag85B, ESAT-6, and the IC31 adjuvant.[22] Dubbed SSI H56-IC31, this vaccine is now being tested in a phase I trial in humans. The trial is being conducted in collaboration with Aeras and is supported by the Bill & Melinda Gates Foundation Grand Challenge #12 consortium.
VPM1002
VPM1002 is a live vaccine made from a genetically modified BCG strain. The vaccine was originally created by the Max Planck Institute for Infection Biology and is now being developed by the company Vakzine Projekt Management. The vaccine has been shown to be safe and immunogenic in a phase Ia trial in Germany and a phase Ib trial in South Africa. The next step is a phase II evaluation of safety and tolerability of the vaccine among HIV-unexposed, BCG-naive newborns in South Africa.[23]
Ad5Ag85A
Ad5Ag85A is another adenovirus-based vaccine that employs serotype 5 (Ad5) as a vector to deliver the antigen 85A. Originally the brainchild of Zhou Xing from McMaster University in Hamilton, Ontario, the rights to further develop and commercialize this candidate were acquired by the Chinese company CanSino in August 2011. CanSino is now partnering with Aeras on this work.[24] A phase I safety and immunogenicity study in BCG-vaccinated and -nonvaccinated healthy adults is under way in Canada.
ID93 + GLA-SE
ID93 + GLA-SE is a new TB vaccine candidate developed by the Infectious Disease Research Institute (IDRI) in Seattle. The construct comprises a recombinant fusion polyprotein including four TB antigens (Rv2608, Rv3619, Rv3620, and Rv1813) delivered together with an adjuvant named GLA-SE. IDRI recently announced a new partnership with Aeras to develop this candidate,[25] and a phase I trial is scheduled to start in June 2012.
Conclusion
The TB vaccine pipeline in 2012 appears relatively healthy but, as in many other areas of research, the global economic downturn is casting a dark cloud of concern over the future. The Treatment Action Group and Stop TB Partnership’s 2011 report on funding trends for TB research and development documented a dismaying 29% drop in vaccine research funding from US$110 million in 2009 to US$78.4 million in 2010. These levels fall woefully short of the estimates contained in The Global Plan to Stop TB 2011–2015, which suggest that US$1.9 billion will be required over this period.[26] Vigorous advocacy will be required in order to address this shortfall, and ensure that an efficacious TB vaccine is developed.
