The science of discovery comes with ethical challenges in human clinical trials
By Richard Jefferys
Over the past several years, there has been a welcome invigoration of the research effort to cure HIV infection. The mainstream media has picked up on this development, and stories about putative or possible cures are appearing more frequently than in the past. Contrary to the impression conveyed by some of these stories, a cure is not likely to announce itself by leaping from a scientist’s test tube waving a flag of victory. To prove their worth, potential curative strategies—whether based on a single approach or a combination—will need to be evaluated in human trials.
The conduct of clinical trials related to HIV cure research raises new issues that multiple stakeholders—regulatory agencies, scientists, biotech and pharmaceutical companies, HIV-positive people, activists, community advisory boards, and institutional review boards—are now beginning to discuss and address. In 2011, TAG along with the AIDS Policy Project, amfAR, and Project Inform sponsored the first workshop on the topic (report available here). More recently, on June 14 of this year, the U.S. Food and Drug Administration (FDA) hosted a one-day public event that sought community input on the regulation of HIV cure research. Webcasts, slide presentations, and a full transcript are available (search “cure research” at fda.org).
Comprehending Risks and Benefits
At this early exploratory stage, the overarching concern involves the risk/benefit calculus of participation in clinical trials. In most cases, there will be risks but little or no possibility of benefit to an individual participant; rather, results from trials will inform and advance the scientific pursuit of a cure. There is universal agreement that this will need to be explained carefully in both educational efforts and the informed consent documents that trial participants are required to read and sign.
Discussions about informed consent in the context of HIV cure research dovetail with wider interest in evaluating how useful and understandable the process is for trial participants. This is a burgeoning area of academic investigation, partly due to concerns that informed consent has become more about protecting against legal liability than providing clear information to individuals joining a trial. Recently published findings on the topic are consistent with the idea that comprehension of informed consent is often far from optimal, and educational interventions and computer-based processes are being assessed as possible solutions. A study by researchers at the HIV INSIGHT network has also suggested that informed consent could be improved by making it an ongoing process throughout a trial, rather than a one-off procedure at the start.
Another potential issue is that the term cure research can lead to a problem known as therapeutic misconception—the desire to be cured might trump cautions pertaining to risk and lack of benefit. In addition to suggesting that informed consent documents be made accessible and easy to understand, those involved in the FDA meeting also endorsed the idea of evaluating how potential trial participants have interpreted the information provided to them before permitting enrollment.
In the absence of any chance of immediate benefit, altruism and the desire to contribute to the search for a cure can still be powerful motivating factors. In a survey of over 2,100 people with HIV that was conducted by David Evans from Project Inform and Nelson Vergel from the Program for Wellness Restoration, over half the respondents (55%) reported that the possibility of benefiting others would motivate them to join a trial even if there were some potential risks. A separate question asked about willingness to participate in studies that might advance the science but offer little prospect of individual benefit; 45 percent responded that they would be either willing or very willing. Additional surveys are now being planned under the aegis of the International AIDS Society’s Towards an HIV Cure initiative.
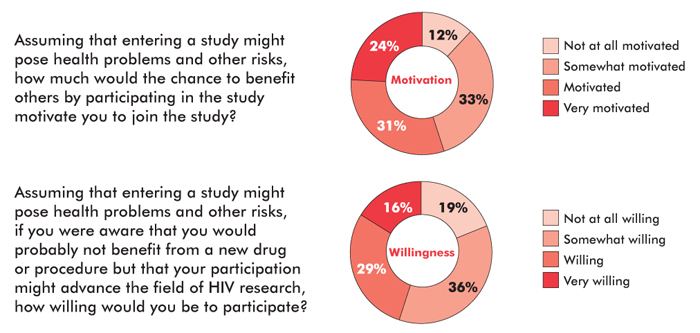
An online community survey of over 2,100 HIV-positive respondents conducted in late 2011 and early 2012 (83% were male, 73% were white, 65% were over 40 years of age, 94% were on ART, and 35% had previously participated in a clinical trial) indicated a high level of altruism regarding early-stage HIV cure research. Adapted from: Evans D. Ethics and informed consent in cure research (Session SUSA28). Paper presented at: workshop, “Towards an HIV Cure”: 19th International AIDS Conference; 2012 July 22–27; Washington, D.C.
Current Examples
Recent and ongoing clinical trials provide examples of the uncertainties and risks that can be associated with cure-related research. A leading approach for awakening the latent HIV that persists despite ART is the administration of anticancer drugs called HDAC inhibitors. Although several HDAC inhibitors are FDA-approved for the treatment of cancers, they have many potential side effects and in some cases score positive in the Ames test (which measures the ability of a drug to cause mutations that might increase the risk of cancers). Three clinical trials assessing the impact of HDAC inhibitors on HIV latency have been conducted to date, fortunately without any serious safety issues emerging. The research has demonstrated that the drugs may be able to activate latent HIV, at least in some infected cells, but no reduction in the overall size of the HIV reservoir has been documented among participants.
The use of ART interruptions in cure-related research offers another example of the potential risks associated with trial participation. Although short-term interruptions were once thought to be relatively benign as long as CD4 T-cell counts did not fall to levels associated with the development of opportunistic infections, the SMART trial showed that viral-load rebound after ART cessation increases levels of inflammation, which is known to pose health risks. In the SMART trial population (in which individuals in one group interrupted ART when their CD4 T-cell counts rose above 350/mm3, then restarted if they fell below 250/mm3), this was associated with significant increase in the risk of illness and death compared with the group that received continuous ART.
As a result of the data from SMART, HIV treatment guidelines explicitly caution against ART interruptions. But if an experimental intervention aims to induce control of HIV off ART (or even to eliminate the virus), the only way of assessing effectiveness is to stop treatment. One proposed approach is to monitor trial participants for viral-load rebound frequently, and restart ART as soon as HIV becomes detectable; this is almost certainly the best way of preventing the virus from provoking high levels of inflammation, but there still could be other concerns such as increasing the size of the HIV reservoir. More controversial—and even less consistent with the current standard of care—are study designs that involve longer-term interruptions or require participants to have stopped ART prior to enrollment. These types of trials are far more likely to increase the risk of inflammation-related morbidity and mortality, and as yet there is no consensus about the acceptable guidelines for trials involving ART interruption.
Thinking of the Children
Adults with HIV infection are not the only population being considered in cure research, and ethical and informed consent issues for studies involving infants and children are even more complex. Currently, a protocol is being developed that intends to investigate whether an apparent cure of HIV in an infant in Mississippi can be repeated. The goal is to identify 20 to 30 infants born to HIV-positive mothers who did not receive ART to prevent mother-to-child transmission, and administer a three-drug therapeutic regimen within 48 hours of birth (instead of the standard two-drug prophylactic approach) until HIV diagnosis is established by testing, which usually takes around seven days. Treatment will then be continued for around three years in infants confirmed to be infected; at that point, if HIV can no longer be detected, ART will be interrupted to assess whether a cure has been achieved. Among the many issues involved will be the incremental increase in risk of side effects that may accompany the use of a three-drug treatment versus a two-drug prophylactic regimen in infants who turn out to be uninfected, and the need to fully explain and discuss the trial with the mothers prior to seeking informed consent (in a situation where time will be limited).
Endpoint Uncertainties
In addition to risks and benefits, another challenge for the regulation of cure research is the selection of appropriate endpoints (the means of measuring the success or failure of an approach). One possible endpoint is the previously cited example of assessing whether viral load rebounds after ART interruption. But for interventions designed to reduce the size of HIV reservoirs, selecting an appropriate endpoint is more challenging due to the difficulties of reliably documenting changes in levels of HIV that are extremely low to begin with. Although a variety of tests for trace amounts of HIV are available, their reliability and comparability is only starting to be assessed, and as yet there is no universally accepted standard technique.
Conclusion
As the HIV cure research effort continues to gain momentum, regulatory and ethical issues will need to be a continuing subject of discussion among all stakeholders. The FDA has expressed a commitment to ongoing engagement on the subject, and a broader dialogue convened by the Forum for Collaborative HIV Research is due to get under way soon. Community advocates and HIV-positive people have an essential role to play in decisions around appropriate risk/benefit, and informed consent and ethics in HIV cure research, and community engagement in discussions around regulatory issues needs to be ongoing. •
Stem Cell Transplantation for People with HIV and Cancers
The first well-documented HIV cure occurred in a now famous individual named Timothy Brown, and resulted from stem cell transplants (SCTs) that were required to treat concomitant acute myeloid leukemia. Two additional possible HIV cures have been reported more recently, also involving SCTs that were administered due to cancer diagnoses (although, unlike Brown, these individuals did not receive transplants from a donor possessing the CCR5-Δ32 mutation that prevents most types of HIV from entering cells).
Understandably, there is a great deal of interest in trying to achieve similar outcomes in other HIV-positive people with cancer who require SCTs, but it’s important to note that there can be regulatory issues associated with these procedures. In some cases, individual approval from the FDA is required, depending on the source of the stem cells (which can be obtained from adults or umbilical cord blood units stored for this purpose) and whether the stem cell source has the CCR5-Δ32 mutation.
As with other types of transplants, a key variable in these procedures is the degree of genetic matching between the stem cell donor and recipient; a poor match typically increases the risk of the transplant’s being rejected and the potentially lethal condition graft-versus-host disease (GVHD). So far, two cases in which people with HIV and cancer received cord blood stem cells from donors with the CCR5-Δ32 mutation have been publicly described: in one case the individual died due to the underlying cancer, and in the other case death occurred due to the development of severe GVHD.
As with other regulatory issues pertaining to cure research, there is a need for a broader public discussion among stakeholders—in this case including experts in stem cell transplantation—about the appropriate guidelines for using this approach to try to cure HIV.
