Poor treatment options spur innovative research strategies
By Polly Clayden, HIV i-Base
Pediatric Drug Development Considerations: Pharmacokinetics
There is an old adage in pediatric medicine: children are not little adults. This is particularly true when it comes to tuberculosis, for which management strategies are largely the same, but dosing guidance and options leave a lot to be desired. Fortunately, a number of initiatives hope to remedy this situation in an effort to reduce global TB mortality among children—currently 100,000 deaths each year.
Advances for adults have ambled over several decades and appropriate drug regimens for TB in children have lagged even further behind. As well as being hard to diagnose, children with TB are usually not infectious—meaning they are rarely considered to be a public health priority. Where children’s needs are not neglected, treatment practice is mostly guided by findings extrapolated from adult research, and so may be inappropriate.
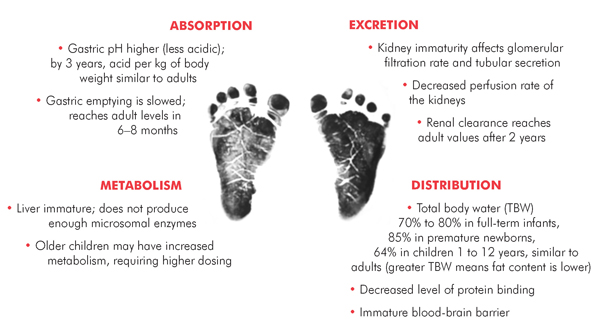
Pharmacokinetics (PK) of all drugs can vary hugely between children and adults because of physiological differences, immaturity of enzyme systems and other mechanisms involved in drug metabolism. There is also great variability across different age groups (see figure).
For treating TB, young children who are unable to swallow tablets need child-friendly formulations. Ideally these should be in solid fixed-dose combination (FDC) forms that are dispersible in liquids and can facilitate dosages across different weight groups.
While scaled-down FDCs using weight-based ratios are available, the World Health Organization (WHO) revised its dosing recommendations for first-line drugs for children in 2010, after several PK studies found suboptimal levels with previously recommended dosages. This means that children are currently treated with far-from-simple mixes of FDCs and divided or single tablets to make up the dosing shortfall. This is not ideal for programs, health workers, or families.
For children who are coinfected with HIV, first-line TB treatment becomes even more complex due to drug-drug interactions between rifampicin and many antiretrovirals (ARVs).
The situation with second-line TB drugs is worse still. There are virtually no data to guide pediatric dosing. Child-friendly formulations are not usually available, and dosages using divided and/or crushed tablets are uncertain. Also, second-line drugs are more toxic than those used in first-line treatment, and adverse events are hard to monitor in children.
For prevention, isoniazid is recommended prophylaxis for TB-exposed infants. However, there are limited data to guide its dosing in neonates and low-birth-weight infants.
What is being done and needs to be done?
Although the current situation for children is a bit bleak, several initiatives, both proposed and ongoing, might offer some improvements in the not-too-distant future.
- New FDCs for first-line treatment are an urgent priority, as are strategies to use them with concomitant HIV treatment. The Global Fund and UNITAID have issued an invitation to manufacturers of TB drugs to submit an Expression of Interest for product evaluation. The generic manufacturer Svizera is developing an FDC using the new WHO dosages. A proposed trial sponsored by the U.K. Medical Research Council (MRC) will look at these, including strategies for children receiving ARVs. They will also see if treatment can be shortened from six to four months.
- For second-line treatment, the University of Stellenbosch, Cape Town, is conducting a large, five-year study to evaluate PK and toxicity of drugs for the treatment and prevention of drug-resistant TB in HIV-positive and -negative children.
- Bedaquiline, recently approved by the FDA for adults, and delamanid, will also need to be formulated and approved for children. Since 2007, all drugs under investigation for adults must have a pediatric investigation plan in order to obtain adult approval from the European Medicines Agency (EMA). The U.S. Food and Drug Administration (FDA) also offers incentives to ensure that pediatric drugs are developed. Importantly, manufacturers must ensure that drugs are submitted to regulators in low- and middle-income countries with a high burden of TB, not just in high-income countries.
- The TB Alliance has proposed a novel approach for speeding access to new TB drugs and regimens in infants and young children. Instead of the traditional approach, which involves sequential PK and safety evaluations in children (from oldest to youngest), the TB Alliance calls for single-dose PK evaluations in hospitalized TB patients in all age groups—both the FDA and EMA are, apparently, open to considering this research strategy. This approach means that approval for the youngest children would not be delayed. It is important, though, that studying the older groups is not delayed if pediatric formulations are unavailable for the younger ones.
- For prevention, the Stellenbosch group has found that isoniazid dosed at 10 mg/kg/day in low-birth-weight TB-exposed infants achieved adult target values. It noted though that the upper range of the WHO-recommended dose (15 mg/kg/day) of isoniazid might be too high for this population.
In order for us to achieve zero TB deaths and suffering in children, TB treatment activists need to understand and highlight these issues in our demands to developers, manufacturers, regulators, and other stakeholders.•
