Conference Call Meeting Minutes
Improving Health Outcomes in HIV Immunologic Non-Responders (INRs)
Orphan Drug Designation Possibilities
A Community Initiated Proposal to the FDA
June 10, 2016
Participants
Jeffrey Murray, M.D., M.P.H., Deputy Director- CDER
Carol Weiss -Section Chief, CBER, Office of Vaccines Research and Review
Ilan Irony – Supervisory Medical Officer, CBER, Office of Cellular Tissues and Gene Therapies
Richard Jefferys- Treatment Action Group
Jeff Sheehy- California Institute of Regenerative Medicine
Jeff Taylor- AIDS Treatment Activists Coalition
Matt Sharp- Treatment Advocate
Lynda Dee- AIDS Action Baltimore
Nelson Vergel- Program for Wellness Restoration
NOTE: No members from the FDA Office of Orphan Products Development (OOPD) were in attendance.
A conference call meeting initiated by HIV community advocates was held with FDA members on June 10, 2016 to discuss ways to advance the evaluation and development of products (drugs or biologics) that demonstrate promise in improving health outcomes in HIV-positive patients with inadequate immune response after long term successful HIV treatment. There is not current research specifically aimed at this population due to real and perceived concerns by industry. Due to the relatively small size of this at-risk population and complexities in proving clinical benefit of potential immune-enhancing therapies, the advocacy community initiated efforts to creatively stimulate industry’s interest by reviewing with the FDA specific approaches to current drug development paths for indications aimed at this population.
To estimate the size and special needs of this patient population, Nelson Vergel gave a slide presentation that contained the following facts and estimates.
Poor CD4 T Cell Recovery Despite HIV Suppression Linked to Increased Morbidity and Mortality
- A subset of HIV-positive people who initiate antiretroviral therapy (ART) and achieve suppression of HIV replication experience poor recovery of CD4 T cell numbers.
- Terms used to describe this subset of individuals include “immuno-virological discordants(ID)” and “immunological non-responders” (INRs).
- As yet, there is no universally accepted definition of INRs (e.g. persistently below 200, 250 or 350 cells despite HIV suppression).
- Depending on the definition, estimates of the proportion of people starting ART who can be categorized as INRs are typically around 5-20%.
- In studies conducted to date, the most consistently reported risk factors for this outcome are low CD4 T cell counts at the time of ART initiation and older age.
- Several published studies have also reported that INRs have a greater risk of morbidity and mortality compared to HIV-positive individuals with more robust CD4 T cell gains.
Functional CD4 Boosting is Essential for Improved INR Survival and Health Outcomes: Statement by INR Researcher
“…to date, strategies to directly influence immune reconstitution by adding interleukin-2 or by modifying cART regimens have failed to show benefit over viral suppression alone; therefore new strategies perhaps aiming at other mechanisms to boost functional CD4 cells or decreasing the levels of immune-activation (e.g. interleukin-7, probiotics) need to be tested in people who show incomplete immune reconstitution despite sustained viral suppression.”
Alexander Zoufaly et al, EuroSIDA in EuroCoord. Immuno-Virological Discordance and the Risk of Non-AIDS and AIDS Events in a Large Observational Cohort of HIV-Patients in Europe. January 31, 2014DOI: 10.1371/journal.pone.0087160
INR Patient Population Estimate
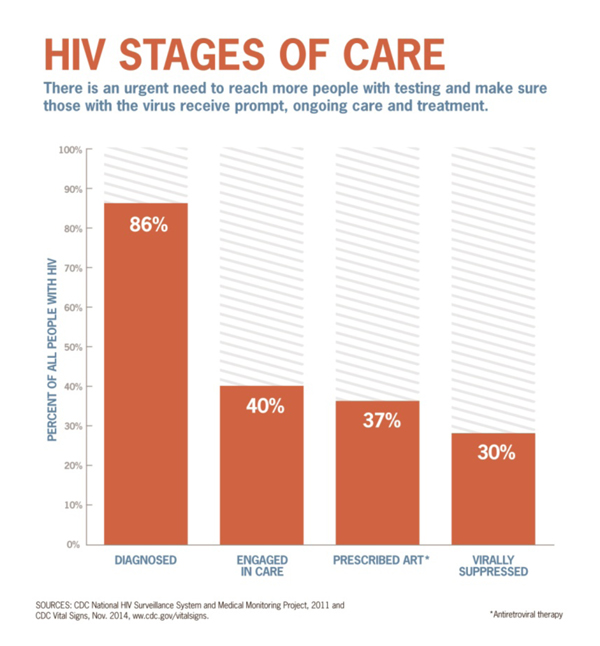
From CDC.gov/vitalsigns
Total HIV Population in the US: 1.3 Million
30% Virally Suppressed = 390,000 people
20% of 390,000 may be INR = 78,000 patients
INR and New Infections: The CDC surveillance report from July 2015 states:
“Among persons with an HIV diagnosis during 2013, 23.6% of infections were classified as stage 3 (AIDS) at the time of diagnosis… The overall percentages were similar for each year during 2009–2013.”
- The actual number in 2013 was 9,846.
- A conservative estimate would be that around 20% of these late-diagnosed individuals might become INRs (2,000 patients/year). This trend may decline in the future depending on outreach/education efforts for treatment and prevention.
- From FDA.gov:
“The Orphan Drug Designation program provides orphan status to drugs and biologics which are defined as those intended for the safe and effective treatment, diagnosis or prevention of rare diseases/disorders that affect fewer than 200,000 people in the U.S., or that affect more than 200,000 persons but are not expected to recover the costs of developing and marketing a treatment drug”.
- Considering current estimate and new infections per year (and excluding death rate of INRs), it would take 61 years to reach 200,000 INR patients in the U.S. (orphan drug designation patient population max)
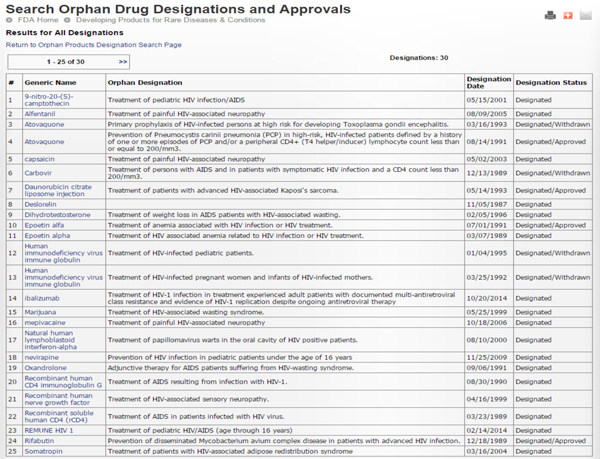
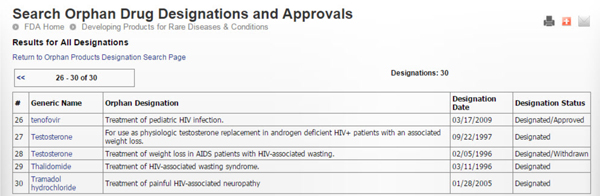
The following tables show the history of HIV/AIDS related orphan drug designations and approvals since the beginning of the epidemic (Source: FDA.gov). Thirty designations and five approvals have taken place. Most approved ones also have more expansive indications. The last orphan drug designation took place on 10/20/2014 for a CD4 monoclonal antibody (ibalizumab) for the treatment of multidrug resistant HIV.
Immunotherapy in HIV infection: Past and Current Challenges
- IL- 2
- IL-2 was extensively studied in several phase II and two large phase III studies. Results from these studies showed that IL-2 significantly increases CD4 counts in the long term. However, this biological effect did not translate into clinical benefit.
- IL- 7
- Cytheris had ambitious plans to conduct a phase III clinical endpoint trial in INRs, but went out of business in 2015. The rights to pursue IL-7 as a therapy for HIV-related immune impairment are reportedly now being directed by a collaboration involving the French National Agency for Research on AIDS and Viral Hepatitis (ANRS) and Cognate BioServices. At best, this will certainly delay evaluation of the ability of IL-7 to reduce morbidity and mortality in INRs.
- SB-728-T (ZFN-CCR5-gene modification)
- Research continues into the use of the Sangamo BioSciences technology to genetically modify CD4+ T cells ex vivo. Like other small companies, Sangamo has not been able to move a product near FDA approval and has shown no interest in pursuing an INR indication after receiving letter from community members advocating for it.
Matt Sharp: Clinical Trial Participant Testimonial
Matt Sharp described his experience as a participant in the clinical trial of SB-728-T that recruited INRs. Sharp was diagnosed HIV-positive in the 1980s and had an extensive antiretroviral treatment history. He was unable to achieve an undetectable viral load until the advent of integrase inhibitors. Despite viral load suppression, CD4 T cell levels remained below 200. Receipt of a single infusion of SB-728-T more than doubled these numbers for a period of years. Sharp also noted this CD4 increase was associated with improved health, specifically an absence of upper respiratory tract infections (URIs) that had previously occurred several times per year. Only recently when CD4 T cell levels close to baseline has he experienced a recurrence of upper respiratory symptoms. Sharp gave a presentation describing his experience at the 2012 International AIDS Conference, which can be downloaded at: http://pag.aids2012.org/PAGMaterial/PPT/30_40/finaldccureworkshop.pptx
Brainstorming on Potential Endpoints
Richard Jefferys briefly outlined several of the key biomarkers that have been associated with morbidity and mortality in HIV infection, particularly in treated individuals and INRs. Although CD4 T cell counts proved to be an inadequate surrogate marker of efficacy in two large trials of the cytokine IL-2, subsequent research suggests that a broader suite of biomarkers might provide more compelling evidence that an intervention is having a disease-modifying effect. Researchers have noted that assessments of immune response functionality might have a particularly important role to play.
- CD4 T cell count
- CD4: CD8 ratio
- Immune response functionality assessed by vaccination
- Inflammatory & coagulation
- Immune activation
- HIV DNA
- T cell phenotypes
- Decay of the HIV DNA reservoir
Nelson Vergel described additional outcomes that could be considered for use as endpoints in clinical trials of candidate interventions for INRs:
- Patient Reported Outcomes (PROs). In 2004, Willke and colleagues reviewed the efficacy endpoints reported in the labels of new drugs approved in the United States from 1997 through 2002 to evaluate the use of patient-reported outcome (PRO) endpoints. Of the labels reviewed, 30% included PROs. Their study aimed to build on this work by describing the current state of PRO label claims granted for new molecular entities (and biologic license applications since February 2006 after the release of the US Food and Drug Administration (FDA) draft PRO guidance. Of the 116 products identified, 28 (24%) were granted PRO claims; 24 (86%) were for symptoms, and, of these, 9 (38%) claims were pain related. Of the 28 products with PRO claims, a PRO was a primary endpoint for 20 (71%), all symptom related. No HIV related indications except one for Egrifta (tesamorelin) has been granted PRO claims. Immune reconstitution could potentiality provide significant improvement in PROs.
- Frailty Index (VACS, MACS, etc). Several frailty indexes validated in HIV related studies could be considered in determining if improved frailty can be a result of immune enhancement therapies. However, we lack data about the incidence of frailty in INR patients.
- Decrease in comorbidites or clinical symptoms (i.e., diarrhea, fatigue, pain, upper respiratory infections, skin disorders, etc). If we can identify the most common comorbidities and health complaints in INR patients, we could specifically design studies focused on monitoring specific symptoms or common illnesses as INR patients acquire better immune response and/or markers. The three FDA members on the call agreed that this may be an approach worth exploring. Community participants will contact the authors of studies assessing morbidity and mortality in INRs to request information on the most frequently observed clinical events.
Regulatory Process – Two Different Tracks
FDA representatives noted that the processes for pursuing an orphan drug designation and a drug approval are separate, involving different departments within the agency. However these processes can be undertaken simultaneously, in parallel. Companies seeking to develop interventions for INRs are encouraged to set up pre-IND meetings with FDA to discuss the issues involved. FDA representatives felt that it was unlikely that marketing approval (whether provisional or full) could be obtained based only on biomarkers or other non-clinical endpoints; it was suggested that including evaluations of non-serious clinical events such as upper respiratory tract infections might offer a means of demonstrating clinical benefit that would not require the trial sample sizes necessary for assessing the impact of an intervention on morbidity and mortality. PROs could potentially be considered – it was highlighted that they have been used in approving influenza treatments.
Action Items:
- Circulate conference call minutes among attendants and interested stakeholders.
- Contact private investigators of previously reported INR cohorts about their knowledge of most common health-related issues seen in INR patients.
- Entertain the possibility of having a meeting with industry, the FDA, advocates and investigators to discuss INR research and opportunities/challenges in the development of therapeutics for this population.
Appendix
Supporting Information and References
Immuno-Virological Discordance (ID) and the Risk of Non-AIDS and AIDS Events in a Large Observational Cohort of HIV-Patients in Europe (EuroSIDA)
- 2,913 patients in EuroSIDA starting at least 1 new ARV with CD4<350 cells/µl and VL>500 copies/mL were followed-up from the first day of VL< = 50 copies/ml until a new fatal/non-fatal non-AIDS/AIDS event.
- Patients were classified over time according to whether current CD4 count > (non-ID) or < (ID) than baseline CD4 count.
- The RR of developing pre-specified non-AIDS events for ID vs. non-ID was 1.96 (95% CI 1.37–2.81, p<0.001). ID was not associated with the risk of AIDS events.
- Long-term mortality in HIV positive individuals virally suppressed for more than three years with incomplete CD4 recovery.
- This purpose of this study was to identify (1) risk factors for failure to achieve CD4 count >200 cells/µL after three years of sustained viral suppression and (2) the association of achieved CD4 count with subsequent mortality.
- Of 5,550 eligible individuals, 835 (15%) did not reach CD4 count >200 cells/µL after three years of suppression.
- Increasing age, lower initial CD4 count, male heterosexual and injection drug use transmission, cART initiation after 1998 and longer time from initiation of cART to start of the virally suppressed period were risk factors for not achieving CD4 count >200 cells/µL.
- Individuals with CD4 ≤200 cells/µL after three years of viral suppression had substantially increased mortality (adjusted hazard ratio 2.60; 95% confidence interval 1.86-3.61) compared to those who achieved CD4 count >200 cells/µL.
- Increased mortality was seen across different patient groups and for all causes of death.
Clin Infect Dis. (2014) doi: 10.1093/cid/ciu038
Lower Current CD4 and Frailty in HIV
- Example of Frailty Related Measures
- Considered frail if 3 or more deficits present:
- Weight loss: ≥10 pounds in past year, self reported and confirmed by physical exam
- Exhaustion: based on responses to two items from the CES-D scale
- Low activity: A modified version of the Minnesota Leisure Time Activities Questionnaire capturing intensity and duration of 18 activities that range from work to child care
- Slowness: Timed 4 m walk
- Weakness: Grip strength measured with dynamometer
Source: Women’s Interagency HIV Study (USA)
Selected Citations for Candidate Biomarkers
CD4 T cell count:
Engsig FN, Zangerle R, Katsarou O, et al. Long-term mortality in HIV-positive individuals virally suppressed for more than 3 years with incomplete CD4 recovery. Clin Infect Dis. 2014 May;58(9):1312-21. doi: 10.1093/cid/ciu038. Epub 2014 Jan 22. http://cid.oxfordjournals.org/content/58/9/1312.full
Zoufaly A, Cozzi-Lepri A, Reekie J, et al. Immuno-virological discordance and the risk of non-AIDS and AIDS events in a large observational cohort of HIV-patients in Europe. PLoS One. 2014 Jan 31;9(1):e87160. doi: 10.1371/journal.pone.0087160. http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0087160
Baker JV1, Peng G, Rapkin J, et al. CD4+ count and risk of non-AIDS diseases following initial treatment for HIV infection. AIDS. 2008 Apr 23;22(7):841-8. doi: 10.1097/QAD.0b013e3282f7cb76.http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3618460/
Additional citations in the Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents chapter on poor CD4 recovery: https://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-arv-guidelines/470/poor-cd4-recovery
CD4:CD8 ratio:
Serrano-Villar S, Sainz T, Lee SA, et al. HIV-infected individuals with low CD4/CD8 ratio despite effective antiretroviral therapy exhibit altered T cell subsets, heightened CD8+ T cell activation, and increased risk of non-AIDS morbidity and mortality. PLoS Pathog. 2014 May 15;10(5):e1004078. doi: 10.1371/journal.ppat.1004078. eCollection 2014 May. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4022662/
Serrano-Villar S, Pérez-Elías MJ, Dronda F, et al. Increased risk of serious non-AIDS-related events in HIV-infected subjects on antiretroviral therapy associated with a low CD4/CD8 ratio. PLoS One. 2014 Jan 30;9(1):e85798. doi: 10.1371/journal.pone.0085798. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3907380/
Lu W, Mehraj V, Vyboh K, Cao W, Li T, Routy JP. CD4:CD8 ratio as a frontier marker for clinical outcome, immune dysfunction and viral reservoir size in virologically suppressed HIV-positive patients. J Int AIDS Soc. 2015 Jun 29;18:20052. doi: 10.7448/IAS.18.1.20052. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4486418/
Immune response functionality assessed by routine vaccination:
Landrum ML, Hullsiek KH, O’Connell RJ, Chun HM, Ganesan A, Okulicz JF, et al. Hepatitis B vaccine antibody response and the risk of clinical AIDS or death. PLoS One. 2012;7(3):e33488. doi: 10.1371/journal.pone.0033488 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3310879/
Valdez H, Mitsuyasu R, Landay A, Sevin AD, Chan ES, Spritzler J, Kalams SA, Pollard RB, Fahey J, Fox L, Namkung A, Estep S, Moss R, Sahner D, Lederman MM. Interleukin-2 Increases CD4+ lymphocyte numbers but does not enhance responses to immunization: results of A5046s. J Infect Dis. 2003 Jan 15;187(2):320-5. Epub 2003 Jan 6. http://jid.oxfordjournals.org/content/187/2/320.full
Valdez H, Smith KY, Landay A, Connick E, Kuritzkes DR, et al. Response to immunization with recall and neoantigens after prolonged administration of an HIV-1 protease inhibitor-containing regimen. AIDS. 2000;14:11–21.http://journals.lww.com/aidsonline/Fulltext/2000/01070/Response_to_immunization_with_recall_and.2.aspx
Inflammatory & coagulation:
Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE, Pedersen C, Ruxrungtham K, Lewin SR, Emery S, Neaton JD, Brenchley JM, Deeks SG, Sereti I, Douek DC; INSIGHT SMART Study Group. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. 2011 Mar 15;203(6):780-90. doi: 10.1093/infdis/jiq118. Epub 2011 Jan 20.http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3071127/
Tenorio AR, Zheng Y, Bosch RJ, et al. Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis. 2014 Oct 15;210(8):1248-59. doi: 10.1093/infdis/jiu254. Epub 2014 May 1.http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4192039/
Rodger AJ, Fox Z, Lundgren JD, Kuller LH, Boesecke C, Gey D, Skoutelis A, Goetz MB, Phillips AN; INSIGHT Strategies for Management of Antiretroviral Therapy (SMART) Study Group. Activation and coagulation biomarkers are independent predictors of the development of opportunistic disease in patients with HIV infection. J Infect Dis. 2009 Sep 15;200(6):973-83. doi: 10.1086/605447.http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2892757/
Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008 Oct 21;5(10):e203. doi: 10.1371/journal.pmed.0050203.http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2570418
Tenorio AR, Zheng Y, Bosch RJ, et al. Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis. 2014 Oct 15;210(8):1248-59. doi: 10.1093/infdis/jiu254. Epub 2014 May 1.http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4192039/
Hsu DC, Ma YF, Hur S, et al. Plasma IL-6 levels are independently associated with atherosclerosis and mortality in HIV-infected individuals on suppressive ART. AIDS. 2016 May 12. [Epub ahead of print] http://journals.lww.com/aidsonline/Abstract/publishahead/Plasma_IL_6_levels_are_independently_associated.97760.aspx
Grund B, Baker JV, Deeks SG, et al. Relevance of Interleukin-6 and D-Dimer for Serious Non-AIDS Morbidity and Death among HIV-Positive Adults on Suppressive Antiretroviral Therapy. PLoS One. 2016 May 12;11(5):e0155100. doi: 10.1371/journal.pone.0155100. eCollection 2016. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4865234/
Borges ÁH, O’Connor JL, Phillips AN, et al. Interleukin 6 Is a Stronger Predictor of Clinical Events Than High-Sensitivity C-Reactive Protein or D-Dimer During HIV Infection. J Infect Dis. 2016 Apr 30. pii: jiw173. [Epub ahead of print] http://jid.oxfordjournals.org/content/early/2016/05/30/infdis.jiw173.abstract
Sereti I, Estes JD, Thompson WL, et al. Decreases in colonic and systemic inflammation in chronic HIV infection after IL-7 administration. PLoS Pathog. 2014 Jan 30;10(1):e1003890. doi: 10.1371/journal.ppat.1003890. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3907377/
HIV DNA:
Ostrowski SR, Katzenstein TL, Thim PT, Pedersen BK, Gerstoft J, Ullum H. Low-level viremia and proviral DNA impede immune reconstitution in HIV-1-infected patients receiving highly active antiretroviral therapy. J Infect Dis 2005;191:348-57. http://jid.oxfordjournals.org/content/191/3/348.full
Chun TW, Shawn Justement J, Pandya P, et al. Relationship between the size of the human immunodeficiency virus type 1 (HIV-1) reservoir in peripheral blood CD4+ T cells and CD4+:CD8+ T cell ratios in aviremic HIV-1-infected individuals receiving long-term highly active antiretroviral therapy. J Infect Dis 2002;185:1672-6. http://jid.oxfordjournals.org/content/185/11/1672.full
Tsiara CG, Nikolopoulos GK, Bagos PG, Goujard C, Katzenstein TL, Minga AK, Rouzioux C, Hatzakis A. Impact of HIV type 1 DNA levels on spontaneous disease progression: a meta-analysis. AIDS Res Hum Retroviruses. 2012 Apr;28(4):366-73. doi: 10.1089/aid.2011.0032. Epub 2011 Aug 30. http://online.liebertpub.com/doi/abs/10.1089/aid.2011.0032
T cell phenotypes:
Weiss L, Letimier FA, Carriere M, et al. In vivo expansion of naive and activated CD4+CD25+FOXP3+ regulatory T cell populations in interleukin-2-treated HIV patients. Proc Natl Acad Sci U S A. 2010 Jun 8;107(23):10632-7. doi: 10.1073/pnas.1000027107. Epub 2010 May 24. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2890853/
Horta A, Nobrega C, Amorim-Machado P, et al. Poor immune reconstitution in HIV-infected patients associates with high percentage of regulatory CD4+ T cells. PLoS One. 2013;8(2):e57336. doi: 10.1371/journal.pone.0057336. Epub 2013 Feb 20. http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0057336
Saison J, Ferry T, Demaret J, et al. Relationship between discordant response to HAART, Tregs, immune activation and low-level viraemia. J Int AIDS Soc. 2014 Nov 2;17(4 Suppl 3):19672. doi: 10.7448/IAS.17.4.19672. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4225367/
Saison J1, Ferry T, Demaret J, M et al. Association between discordant immunological response to highly active anti-retroviral therapy, regulatory T cell percentage, immune cell activation and very low-level viraemia in HIV-infected patients. Clin Exp Immunol. 2014 Jun;176(3):401-9. doi: 10.1111/cei.12278. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4008985/
Overall review (from 2009):
Gazzola L, Tincati C, Bellistrì GM, Monforte Ad, Marchetti G.. The absence of CD4+ T cell count recovery despite receipt of virologically suppressive highly active antiretroviral therapy: clinical risk, immunological gaps, and therapeutic options. Clin Infect Dis. 2009 Feb 1;48(3):328-37. doi: 10.1086/595851. Review http://cid.oxfordjournals.org/content/48/3/328.full
