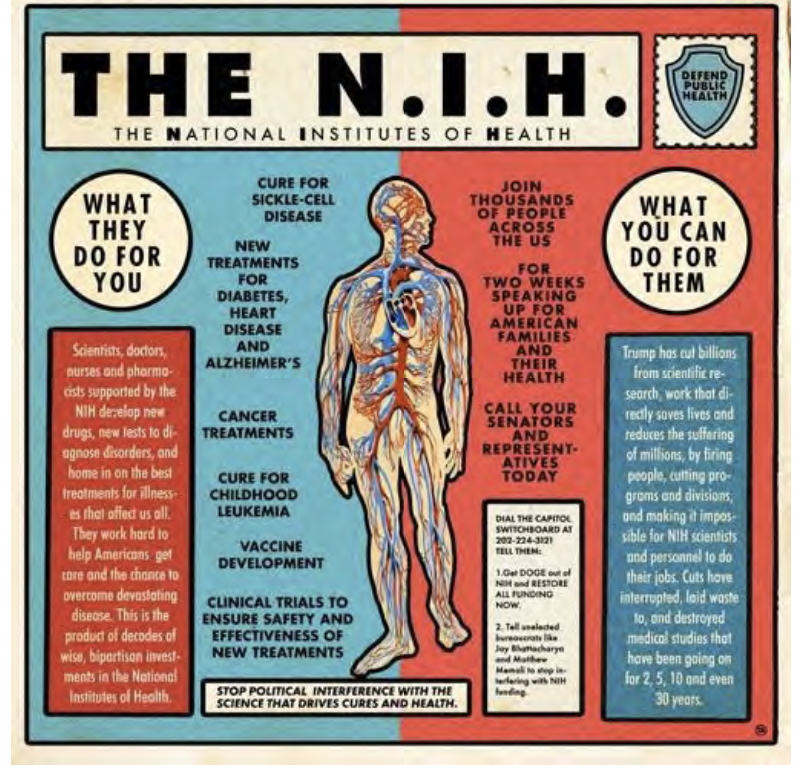
The Research Matters Advocacy Toolkit is a vital resource for researchers committed to advancing health equity and biomedical advocacy. Developed by TAG, HIVMA, and AVAC, this toolkit provides key messages, practical advocacy guides, and essential resources to help mobilize researchers and research advocates advance our collective efforts to protect and accelerate HIV and TB science amidst an increasingly hostile policy landscape. Since January 2025, faced with the wide scale decimation by the U.S. government of critical research activities in the U.S. and globally, advocates and researchers are leading efforts to mitigate and reverse the harms to progress in HIV and TB research.
This toolkit was developed to provide HIV and TB researchers information, messages, opportunities and guidelines to assist with local and national advocacy efforts around restoration of NIH funding for research. Whether engaging in policy discussions, mobilizing communities, leading or participating in protests/civil disobedience actions, or shaping research priorities, this toolkit equips you with the tools to make a meaningful impact. Explore the full toolkit and join the movement to protect science that saves lives.