June 2016
By Suraj Madoori
Edited by Erica Lessem and Kenyon Farrow
The End of TB Starts with Science
Eliminating tuberculosis (TB), the leading infectious killer in the world, will not happen without the critical leadership of the United States. Innovative policymaking underpinned by catalyzing science has led to a dramatic reduction in the number of new TB cases each year in the country. Yet TB is still far from global elimination, and an uptick in the number of new domestic TB cases in 2015 points to the fragility of progress. The bold scale-up of policymaking in the United States requires an equally bold parallel strategy to increase government investment in research and development (R&D) for new tools, especially to take on the latest emerging battle against drug-resistant TB.1 Indeed, the White House’s 2015 National Action Plan to Combat Multidrug-Resistant Tuberculosis includes a strong focus on R&D. To enable the plan’s execution, and to support robust TB R&D for new tests, treatments, and vaccines, at least $300 million by FY 2017 and $400 million by FY 2020 is needed in public funding from the United States.
The path to the end of TB starts with science, and history substantiates this strategy. Since the first antiretroviral medications were introduced in the mid-1990s in response to the HIV crisis, a dramatic public and private investment in science has occurred. This catalyzed a pipeline of cutting-edge treatments that reduced toxic, multiple-pill treatments to daily single-pill regimens, effectively making HIV a manageable, chronic condition. Additionally, several recent blockbuster cures may now level the battle against hepatitis C virus.
In the early days of antibiotic therapy, science spearheaded huge progress against TB, providing an evidence-based cure that was much more effective than the previously prescribed rest and sunlight. Cutting-edge research efforts in the 1950s to prevent TB helped curb the epidemic in vulnerable populations.2 Yet since then, TB R&D has declined dramatically, and no new drug class has been developed for over 40 years. Even with a recent resurgence in TB R&D in the past 15 years, inspired by numerous successful investment models from other disease treatment areas, TB remains woefully behind with outdated tests; no broadly protective vaccine; and lengthy, toxic treatments with daily multiple pills or painful injections. New options to diagnose, prevent, and treat TB are finally in the pipeline, but insufficient funding has stalled their urgently needed advancement to give us effective tools to support the global health security agenda against TB.
This policy brief provides an overview of investments made by the U.S. government and explains how increasing TB R&D funding can catalyze the development of better vaccines, diagnostics, and treatments for TB. It is built upon resource tracking and reporting done by Treatment Action Group (TAG),3 and it recommends areas in which investments will not only fill research gaps but also provide clarity in the vision proposed by the National Action Plan and prevent the rising threat of multidrug-resistant TB (MDR-TB).
Flatlined Since 2009: U.S. Science Purchasing Power and Parity in TB R&D
The U.S. government leads the world in committing resources to TB research, contributing 37% of the $674.0 million in global funding in 2014. However, its allocation for TB has stagnated since 2009 and has fallen in terms of purchasing power as inflation erodes the value of flatlined investments.* To maintain previous levels of research support in real terms, nominal funding must increase (Figure 1).4
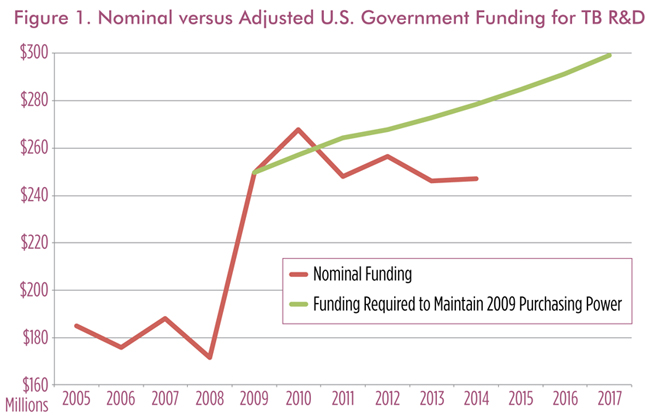
Overall, TB R&D is woefully underfunded, with a gap of $1.3 billion to meet the funding targets set by the Global Plan to Stop TB.5 As the leader in TB R&D funding, the United States should also lead in closing this gap. Without increased investments, we will continue to struggle to control TB with inadequate vaccines, diagnostics, and treatments. Current research may be discontinued, and future research may never begin.
*Funding target methodology: TAG measures funding annually since 2005 across six areas of TB R&D: basic science, diagnostics, drugs, vaccines, operational research, and infrastructure projects. We calculated funding targets in this brief by applying measures of inflation from the Biomedical Research and Development Price Index (BRDPI) to the data we collected. This index measures the average change in prices of research-related goods and services (e.g., personnel, supplies, and equipment) purchased with the NIH budget. The annual change in the BRDPI indicates how much research spending must change to maintain purchasing power (i.e., to maintain the same level of research activity) at the previous year’s level.
The Beginning of the End of TB: Building on Successful Investments
TB R&D investments to date from various U.S. agencies have been critical in making global headway, modeling public–private partnerships, establishing vital research networks, and creating a track record of impressive achievements in the pipeline.
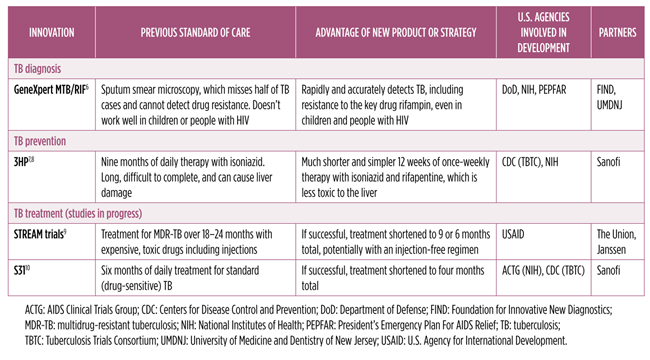
Table 1 summarizes the history of developments in diagnostics and prevention that were made possible through the critical public funding role of the U.S. government. But unlike HIV and hepatitis C virus research, TB treatment has had too few new breakthrough options because of lack of R&D funding. Modest, yet critical, increases to the current commitments in the next three fiscal years will build upon these achievements, scale up the pipeline, save money, and accrue benefits for public health in globally ending TB.
Breathing Life: Priority Recommendations for the Scaling Up of TB R&D Investment
The following recommendations call for discrete funding targets and disbursement of additional TB R&D funding for key federal agencies contributing to the pipeline of new tests, treatments, and vaccines:
Increase funding levels to $300 million by FY 2017: Increasing spending from $247.0 million to $300 million on TB R&D in 2017 would allow the U.S. government to keep pace with rising costs of biomedical research and maintain purchasing power with 2009. However, this funding would not represent an increase in real funding, but just enough to keep pace with inflation. A $53-million increase in funding could, for example, be disbursed across key agencies supporting TB R&D as follows†:
- NIH: $17.0 million
- USAID: $15.0 million
- CDC: $16.0 million
- The Food and Drug Administration (FDA): $5.0 million
- Additional increases to support TB research at the DoD, National Science Foundation (NSF), and Biomedical Advanced Research and Development Authority (BARDA)
Increase funding levels to $400 million by FY 2020: A yearly average increase of $33.3 million to reach $400 million on TB R&D in 2020 will allow investments to outpace the rising costs of biomedical research. This will also increase funding for TB R&D by $72 million in real terms over 2009.
†These reflect the need to increase funding for various types of research sponsored by different U.S. agencies, from the basic science the NIH supports to clinical trials sponsored by USAID, the CDC, and the NIH, and epidemiological research from the CDC; to orphan drug development and regulatory strengthening that the FDA supports; to other research supported by the DoD, the NSF, and BARDA.
Implications of Improved Funding for TB R&D
Increasing TB R&D funding, as well as taking subsequent global action on TB R&D, would provide a number of distinct advantages for the U.S. government:
- Strengthening global health security and preparedness: In light of the lessons from the Zika virus and Ebola response, new strategies and effective tools gained through increased TB R&D will save money and strengthen global health security and preparedness—especially in areas that are seeing rising rates of MDR-TB.
- Leveraging outside funding: U.S. government contributions help attract additional investments in TB R&D, from industry partners to foundations to other countries. Calling upon the pharmaceutical industry, European Union, and BRICS (Brazil, Russia, India, China, South Africa) nation governments to increase their contributions to be in line with U.S. investments will move us toward a more shared global health security agenda on TB elimination.
- Building American science power: More investment on the U.S. side in TB R&D can have profound effects on building American science power on a pertinent global issue and encourage scientists to either stay or enter the sector. Flat TB R&D funding has a major impact on the pool of researchers, with many forgoing opportunities to do much-needed TB research because of limited funding. The continued plateauing of public investments—or worse, sequestration or cuts—will jeopardize and delay research; the Tuberculosis Trials Consortium had to close some clinical trial sites because of funding cuts, and this slows down enrollment and study results.11 Additionally, the lack of novel diagnostics, treatments, and vaccines also presents an opportunity for young American scientists to fill that gap in the pipeline. Increasing R&D funding will be the spark a young generation of scientists needs to prioritize this critical global health issue. Lastly, new investments can greatly help current and future studies such as those coordinated by the Tuberculosis Trials Consortium through expanding trial sites, increasing enrollment into studies, and paving the way for more studies that are currently in a holding pattern without funding.
- Achieving the National Action Plan to Combat MDR-TB: With the release of the National Action Plan in 2015, U.S. leadership has acknowledged MDR-TB as a crucial domestic concern. Implementation of the plan, however, is limited by budgetary constraints.12 Yet it articulates the exact science agenda needed to tackle this problem with directives to the NIH, USAID, the CDC, and other federal agencies on building capacity for clinical research and evaluation under the goal to “Accelerate Basic and Applied Research and Development to Combat Multidrug-Resistant Tuberculosis.”13 With the agenda already defined by the U.S. government, appropriating greater targeted funding for TB R&D will help make this ambitious plan a reality for both American and global communities affected by TB.
ENDNOTES
- Sun LH. “TB cases increase in U.S. for first time in 23 years” The Washington Post [Internet]. 2016 March 24. Available from: https://www.washingtonpost.com/news/to-your-health/wp/2016/03/24/tb-cases-increase-in-u-s-for-first-time-in-23-years/. (Accessed 2016 June 3)
- Comstock GW, Baum C, Snider DE Jr. Isoniazid prophylaxis among Alaskan Eskimos: a final report of the bethel isoniazid studies. Am Rev Respir Dis. [Internet]. 1979 May [cited 2016 June 3];119(5):827–30. Available from: http://www.ncbi.nlm.nih.gov/pubmed/453704.
- Frick M. Treatment Action Group. 2015 report on tuberculosis research funding trends, 2005–2014: a decade of data. Edited by Andrea Benzacar, Mark Harrington, and Erica Lessem. New York: Treatment Action Group; 2015. Available from: https://www.treatmentactiongroup.org/sites/g/files/g450272/f/201511/TB_FUNDING_2015_WEB.pdf. (Accessed 2016 May 25)
- Ibid.
- Stop TB Partnership and World Health Organization. The Global Plan to Stop TB: 2011-2015. Geneva: World Health Organization; 2011. Available from: http://www.stoptb.org/assets/documents/global/plan/TB_GlobalPlanToStopTB2011-2015.pdf. (Accessed 2016 May 26)
- Jervis, C. GeneXpert rapid TB test price reduced in historic agreement. TAGline. 2012 Fall. Available from: https://www.treatmentactiongroup.org/tagline/2012/fall/genexpert-rapid-tb-test-price-reduced-historic-agreement. (Accessed 2016 May 25)
- Shepardson D, Marks SM, Chesson H, et al. Cost-effectiveness of a 12-dose regimen for treating latent tuberculous infection in the United States. Int J Tuberc Lung Dis. 2013 Dec [cited 2016 May 25];17(12):1531–7. Available from: doi: 10.5588/ijtld.13.0423.
- Martinson NA, Barnes GL, Mouton LH, et al. New Regimens to Prevent Tuberculosis in Adults with HIV Infection. N Engl J Med. [Internet]. 2011 July [cited 14 July 2016]; 365:11-20. Available from: http://www.nejm.org/doi/ref/10.1056/NEJMoa1005136#t=abstract.
- International Union Against Tuberculosis and Lung Disease. Clinical Trials – Current Trials: STREAM clinical trial to test first all-oral MDR-TB treatment regimen [Internet]. (date unknown) (cited 2016 June 4). Available from: http://www.theunion.org/what-we-do/research/clinical-trials.
- ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (U.S.). 2015. Identifier NCT02410772, TBTC Study 31: Rifapentine-containing tuberculosis treatment shortening regimens (S31/A5349); 2015 February 8 (cited 2016 June 1). Available from: https://clinicaltrials.gov/ct2/show/NCT02410772.
- Treatment Action Group. The tuberculosis trials consortium: linking innovative research with TB care & treatment! New York: Treatment Action Group; 2014. Available from: https://www.treatmentactiongroup.org/sites/g/files/g450272/f/201311/TBTC%20Advocacy%20Backgrounder.pdf. (Accessed 2016 June 2)
- White House (U.S.). National action plan for combating multidrug-resistant tuberculosis. Washington: The White House; 2015 December. Available from: https://www.whitehouse.gov/sites/default/files/microsites/ostp/national_action_plan_for_tuberculosis_20151204_final.pdf. (Accessed 2016 May 23)
- Ibid.
