The goal of hepatitis C virus (HCV) treatment is a cure (when there is no hepatitis C virus in a person’s bloodstream at least 12 weeks after treatment is finished).
What is Zepatier?
Zepatier is two hepatitis C virus-fighting drugs in one tablet. These drugs block different steps of the virus lifecycle. In the United States, Zepatier is approved for people who are over 18 years old with hepatitis C genotype 1 or genotype 4.
How is Zepatier used?
Zepatier is a beige pill, taken once daily, with or without food, for 12 or 16 weeks. Some people will need to take another drug with Zepatier, called ribavirin (RBV), twice daily.
Zepatier comes in a 14-pill blister pack; each pill is individually packaged to protect Zepatier from moisture. Keep Zepatier in the packaging until you take it.
Drug resistance testing is recommended for people who have hepatitis C genotype 1a before starting treatment with Zepatier. Some people with genotype 1a will need 16 weeks of treatment and RBV (see What is drug resistance?, below). It is important to make sure that you have gotten the right treatment (with or without RBV), for the recommended length of time (12 or 16 weeks).
FDA Recommendations and Cure Rates for Zepatier in HCV Genotypes 1 or 4
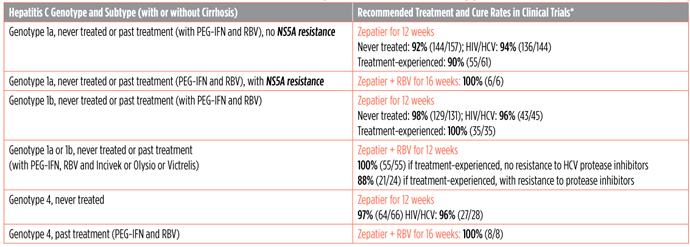
*Cure rates in clinical trials are higher than in real life since the people in them are usually healthier and get extra monitoring and support.
The most important thing a person can do to be cured is not to miss taking doses of HCV treatment—this is called adherence. Adherence lowers the risk for drug resistance.
What is drug resistance?
Each day, HCV makes billions of copies of itself. Some of these copies are not exactly the same as the original virus. They may have changes (called mutations) that can stop hepatitis C drugs from working. If people miss doses of their treatment, HCV gets a chance to reproduce—and some of the copies it makes may not respond to HCV treatment.
Some people have drug resistance even though they have never been on hepatitis C treatment—but many can be cured anyway.
Pretreatment resistance testing is recommended for people with HCV genotype 1a because they may have certain mutations that make Zepatier less effective. This can be overcome by taking Zepatier for 16 weeks with RBV.

Most people who are not cured have resistance to one or more of the HCV drugs they’ve taken. Resistance to certain hepatitis C drugs can disappear within months. But resistance to other drugs can last for years and might prevent re-treatment from working.
Side effects from Zepatier:
Make sure to talk with your health care provider about possible side effects and how they will be managed. In clinical trials, the most common side effects from Zepatier were fatigue and headache, nausea, insomnia, and diarrhea. People taking RBV also experienced anemia, shortness of breath, rash, itching, depression, irritability, and achy joints (see TAG’s ribavirin (RBV) fact sheet for more information). Most of these side effects were mild.
Liver enzyme levels may increase while taking Zepatier.
Your health care provider should check your liver with blood tests before you start taking Zepatier and 8 weeks after starting treatment. For people taking Zepatier for 16 weeks, another blood test is recommended at 12 weeks after starting treatment.
In clinical trials, people over 65 years old, women, and people of Asian ancestry had higher levels of Zepatier in their bloodstream and were more likely to have liver enzyme elevations.
Zepatier and age, gender, and race/ethnicity:
In clinical trials, cure rates did not differ by age (over 65 vs. under 65). Cure rates have been the same for women and men, and in all races. Information is limited because most of the people in the trials were white.
Does Zepatier work for HIV-positive people?
Zepatier works just as well for coinfected people. In C-EDGE Coinfection, a 189-person trial, 95% (179/189) of people being treated for the first time were cured. In the C-EDGE Treatment-Experienced trial, cure rates among people with HIV/HCV were 100% (11/11) after 12 weeks of Zepatier
(with or without RBV). Cure rates among people treated with 16 weeks of Zepatier were 83% (5/6) without RBV and 100% (4/4) with RBV.
Zepatier and other medications:
Zepatier should not be used with certain drugs. Combining medications can increase or lower drug levels (called drug-drug interactions). Increasing drug levels can make side effects from each drug worse. If drug levels get too low, a drug can stop working, putting people at risk for drug resistance or not being cured.
Talk with your health care provider about starting or stopping any medications, supplements, or herbal remedies.
Some drugs should be switched, stopped, or avoided while using Zepatier. More information is available in prescribing information for Zepatier online at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2016/208261Orig1s000lbl.pdf and www.hep-druginteractions.org.
Zepatier and HIV antiretrovirals:
Zepatier can be used with these HIV drugs: Complera/Edurant, Emtriva, Epivir, Isentress, Tivicay, Triumeq, Truvada, Viread, and Ziagen.
Zepatier during pregnancy, nursing, and in children:
It is not known whether Zepatier causes harm to unborn babies. If you are pregnant, or planning pregnancy, talk with your health care provider about the risks and benefits of HCV treatment.
It is not known whether Zepatier passes into breast milk or whether nursing during treatment with Zepatier causes harm to infants and children.
Zepatier has not been studied in children and is not approved for people under 18 years old.
Ribavirin causes birth defects, and it can be fatal to unborn babies. Ribavirin should not be used by pregnant women or by male partners of pregnant women. Ribavirin stays in a person’s body for months. Women and their male partners should avoid pregnancy for six months after they have stopped taking ribavirin. Using two forms of birth control to prevent pregnancy while taking ribavirin—and for six months afterward—is recommended. Nursing during treatment with ribavirin is not recommended.
There is a ribavirin pregnancy registry at: http://www.ribavirinpregnancyregistry.com.
Storing Zepatier:
Keep Zepatier at room temperature (below 68°F to 77°F).
Zepatier in people with kidney disease:
Zepatier can be used without dose adjustment in mild, moderate, or severe kidney disease, including people on dialysis. In C-SURFER, a clinical trial of people with stage 4 and stage 5 kidney disease, 94% (115/122) were cured by 12 weeks of Zepatier.
Zepatier in people with cirrhosis:
Hepatitis C treatment guidelines recommend that people with serious liver damage (Child-Pugh Class B or C cirrhosis) be treated by a specialist. People with Class B and Class C cirrhosis should not use Zepatier.
Zepatier can be used by people with mild liver impairment (Child-Pugh Class A cirrhosis).
Access to Zepatier
Access may be restricted by public and private payers. The criteria differ by type of coverage and the state it was issued in. Merck’s Access Program may help people who have private insurance. Information is available at: http://www.merckhelps.com/ZEPATIER. Enrollment forms are available online at: http://www.merckhelps.com/docs/MAP_Enrollment_Form_INFC-1161739_English.pdf or by phone at 1.866.251.6013, Monday through Friday, from 8 a.m. to 8 p.m. (Eastern Time).
Some uninsured people may be eligible for free medication through the Merck Patient Assistance Program; information is available online at: http://www.merckhelps.com/programs.aspx?tab=MAP and by phone at 1.800.727.5400, Monday through Friday from 8 a.m. to 8 p.m. (Eastern Time).
This fact sheet is current as of April 2016. Always check for updated information.

