Updated April 2016
What is Daklinza?
Daklinza (daclatasvir) is an HCV-fighting drug that blocks different steps of the virus life cycle. In the United States, Daklinza is approved for people over 18 years old who have HCV genotype 1 or genotype 3 (although it has been used in other genotypes).
How is Daklinza used?
Daklinza is taken once daily with another drug, called Sovaldi. These drugs can be taken with or without food, for 12 or 24 weeks. Daklinza and Sovaldi have been used in HCV genotypes 1, 2, 3, and 4 (including in people with HIV/HCV coinfection) and before and after liver transplantation.
People with cirrhosis may need longer treatment with Daklinza and Sovaldi, or a third drug called ribavirin (RBV), which is taken twice daily with food.
Resistance testing is also recommended for all people with HCV genotype 1 who were not cured by Daklinza (or other drugs from the same family, such as Harvoni or Zepatier); treatment should be tailored to the results.
Daklinza: Recommendations from the FDA, and Cure Rates*
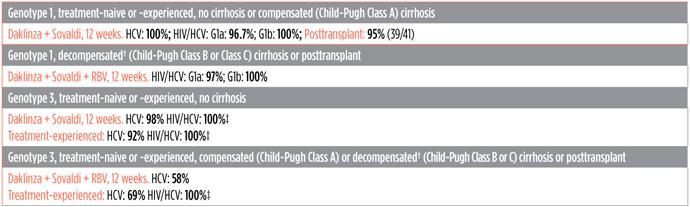
*In people with decompensated cirrhosis or transplant recipients, start RBV at 600 mg/day; increase to 1,000 mg/day as tolerated.
†Cure rates in clinical trials are higher than in real life since the people in them are usually healthier and get extra monitoring and support.
‡Studied in fewer than 10 people
The most important thing a person can do to be cured is to not miss taking doses of HCV treatment—this is called adherence. Adherence lowers the risk for drug resistance.
What is drug resistance?
Each day, HCV makes billions of copies of itself. Some copies are not exactly the same as the original virus. They may have changes (called mutations) that can stop hepatitis C drugs from working. If people miss doses of their treatment, hepatitis C gets a chance to reproduce—and some of the copies it makes may be resistant to HCV treatment.
Some people have drug resistance even though they have never been on hepatitis C treatment—but many can be cured anyway. Certain mutations make Daklinza less effective, including one called Y93H.
Daklinza and Sovaldi in Genotype 3, with and without the Y93H Mutation
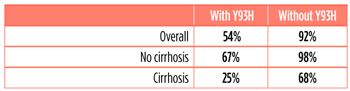
Most people who are not cured by HCV treatment have resistance to one or more of the drugs they’ve taken. Resistance to certain hepatitis C drugs can disappear within months. But resistance to certain drugs, including Daklinza, can last for years—and might limit re-treatment options.
Daklinza and age, gender, and race/ethnicity:
In clinical trials, cure rates did not differ by age (over 65 vs. under 65). Cure rates have been the same for women and men. In ALLY-2, a trial of Daklinza and Sovaldi in people with HIV/HCV, the overall cure rate was the same regardless of race/ethnicity. Information about how well Daklinza works by race or ethnicity is limited since most people in the clinical trials were white.
Side effects from Daklinza:
Talk with your health care provider about possible side effects and how they will be managed. In clinical trials of Daklinza and Sovaldi, the most common side effects were fatigue and headache; usually, these were mild.
Does Daklinza work for HIV-positive people?
Yes. In ALLY-2, a clinical trial in 153 HIV/HCV-coinfected people, 149 (97%) were cured after 12 weeks of Daklinza and Sovaldi.
Daklinza and other medications: drug-drug interactions:
Daklinza should not be used with certain drugs. Combining medications can increase or lower drug levels (called drug-drug interactions). Increasing drug levels can make side effects from each drug worse. If drug levels get too low, a drug can stop working, putting people at risk for drug resistance or not being cured.
Talk with your health care provider before starting or stopping any medications, supplements, or herbal remedies.
Some drugs should be switched, stopped, or avoided while using Daklinza.
Sovaldi—which is used with Daklinza—should not be used with a medication called amiodarone because it can cause life-threatening heart problems.
More information is available in Daklinza’s prescribing information (www.accessdata.fda.gov/drugsatfda_docs/label/2015/206843Orig1s000lbl.pdf) and at: www.hep-druginteractions.org.
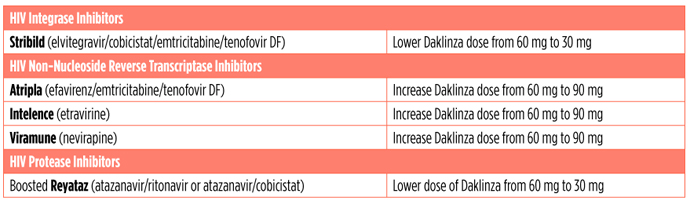
Daklinza and HIV antiretrovirals:
Daklinza can be used with most HIV drugs. A lower or higher dose of Daklinza may be needed when it is used with certain antiretrovirals.
Storing Daklinza:
Keep Daklinza at room temperature (between 59°F and 86°F).
Daklinza in people with kidney disease:
Daklinza can be used without dose adjustment in people with mild, moderate, or severe kidney disease.
Daklinza in people with cirrhosis:
HCV treatment guidelines recommend that people with serious liver damage (Child-Pugh Class B or C cirrhosis) be treated by a specialist. Daklinza can be used in mild, moderate, or severe hepatic impairment without dose adjustment. In clinical trials, people with Child-Pugh Class B or Class C cirrhosis have been treated with Daklinza and Sovaldi, with or without RBV. Daklinza-based treatment is less effective for people with Child-Pugh Class C cirrhosis.
Daklinza and Sovaldi have also been used to treat people for hepatitis C after liver transplantation.
Daklinza during pregnancy, nursing, and in children:
It is not known whether Daklinza causes harm to unborn babies. In animal studies of pregnant rats and rabbits, very high doses of Daklinza caused birth defects, miscarriage, and maternal death. No harm was seen at lower doses.
If you are pregnant or planning pregnancy, talk with your health care provider about the risks and benefits of HCV treatment. It is not known whether Daklinza passes into human breast milk (in animal studies using much higher doses, it was found in the breast milk of rats).
Daklinza has not been studied in children, and it is not approved for people under 18 years old.
Access to Daklinza
Access may be restricted by public and private payers. The criteria differ depending on the type of coverage and the state it is issued in. Patient Support Connect is BMS’s patient assistance program for Daklinza. People with private insurance may be eligible for assistance with copayments. Uninsured people may be eligible for medication at no charge. Information and enrollment forms are available online at: https://bmsdm.secure.force.com/patientsupportconnect/patient and http://www.bmspaf.org/documents/bmspaf-enrollment-form.pdf or by phone at 1.800.736.0003.
This fact sheet is current as of April 2016. Always check for updated information.

