Bungled T-20 Launch Claims Its First Casualties, as Losses Spread
The Year of Fuzeon: Trimeris Daily Share Price — Launch to Lability
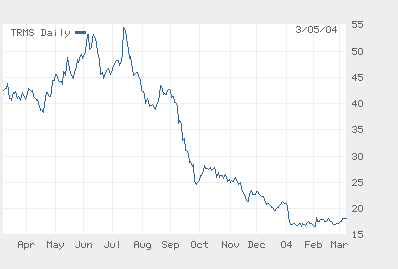
Source: Wall Street Journal
Curse of the “Early Terminator”
This month marks the one-year anniversary of the FDA licensure for T-20 (Fuzeon). The much anticipated and storied therapeutic received universal acclaim, as did its sponsor, for innovation and perseverance. Following a tortuous and sometimes controversial clinical development, the first in a new class of antiretrovirals generically lumped together as entry inhibitors, fêted its official launch at the IAS conference in a sultry Paris last July. And despite prolonged clamoring of shortages and lavish predictions of early blockbuster status, uptake proceeds at a moribund pace — and Trimeris finds itself not awash in cash but flummoxed by a vanishing “footprint” and soaring marketing expenses. One year on, rather than their barn storming potion flying off the shelves like the better built mousetrap it was billed to be, they find themselves sitting on warehouses full of the stuff, fretting about how much they’re now going to have to spend to turn the tide. Mike Barr prepared this special report for TAGline.
It may seem ages ago that activist outrage at the production limitations (and usurious price) of the much anticipated T-20 erupted. Resourceful physicians called in their chits with those in positions of power in order to ensure supply as Fuzeon distributor ChroniMed first rolled into town. And in anticipation of Medicaid snafus and red tape, ADAP coordinators urged physicians to place orders for the drug well in advance of actual prescribing decisions. Such was the fury of Fuzeon. A textbook case of triumphant “expectations management” on the part of the Trimeris/Roche marketing team.
At the July International AIDS Society (IAS) conference in a sultry Paris (where later it was discovered that over 10,000 “plus âgés” died of the record heat while ER staff were sprawled out on Mediterranean beaches or cooling themselves in quaint alpine villages), 2003 was trumpeted as “the year of Fuzeon.” And according to even the most conservative prognostications, Fuzeon was set to become the true billion-dollar drug — a milestone which had eluded the eponymous molecule of Vertex/Glaxo years earlier (see Barry Werth, 1994).
Great furor ensued when, shortly after its U.S. FDA approval, Trimeris/Roche announced there would be sufficient quantity of drug for only 8,000-10,000 of the neediest cases — down from an earlier announcement of 12,000-15,000. Four months later the company had curiously reversed itself again, saying it would have drug enough for 18,000 patients worldwide — and expected to be able to expand that to 24,000-32,000 in 2004 and 40,000 in 2005.
Looking back, this all may seem a little absurd. For as of year-end 2003 the company had provided Fuzeon to all of 4,350 patients — and actually sold it to fewer than 4,000. (Some 1,300 Fuzeon “kits,” ~430 patients, were distributed via the Trimeris Patient Assistance Program or “PAP” and thus need to be subtracted from the raw sales figures.) And with a 25% “early terminator” rate (patients who unexpectedly discontinue the drug within the first 1-2 months), that number was expected to fall significantly (to somewhere around 2,800 patients) by the time the ink had dried on the quarterly report. From Trimeris predictions of 8,000-10,000 patients on Fuzeon by year-end 2003, the stark reality was that they had fallen short by over half. 2003 sales figures, initially estimated at $120 million — and expected to peak around $1 billion, came in at a mere $36 million. The question on everyone’s mind: what went wrong?
In six short months the Trimeris/Roche team has alienated its former Wall Street champions, furloughed a third or so of its North Carolina research staff, terminated a relationship with its principal distributor (ChroniMed), liquidated what remained of its R&D portfolio and lost a cool $860M in market cap in the process. Greed may be good, Gordon Gekko, but the Trimeris story nearly renders comprehensible the old saw about shooting oneself in the foot to spite one’s face.
On a more practical level, the pity here is that most of Trimeris’ misfortune was entirely avoidable. And while it is tempting to bask in the bittersweet revenge that appears to have wrought havoc on Trimeris/Roche’s quick road to riches, Trimeris’ failure is good for virtually no one. An entirely unsuccessful product launch of the first HIV therapeutic with a truly novel mechanism of action would likely send shock waves through the entry inhibitor portfolios of other upstarts. Still, there are clearly lessons to be learned — and then not repeated. One may never know for sure how pricing and early access decisions were made — how real the supply concerns, how costly the drug development — but in retrospect, these two decisions appear to have been pivotal.
A real expanded access program may have helped them to avoid the most serious problems they now face: physician comfort with prescribing the drug, broader patient “uptake,” and nursing support. While it may have risked exposing too many “real life” salvage patients to a product that was shown to shine in the fastidiously managed TORO studies, this was probably a bet worth wagering. Even if a bit of the buzz had been beaten back by a Fuzeon failure story here or there, an ample expanded access program early on would have also “cleared the deck” of the neediest cases and paved the way for a more ecstatic word of mouth once the product was actually on pharmacy shelves. In this respect at least, the 25% early discontinuation rate which Trimeris has experienced since launch need not have been so high.
Then, of course, there is the issue of price. Eyebrows will always be raised when the price of a desperately needed new therapeutic comes in at just a few thousand dollars above what the market currently bears. Cynical suspicions aside, one cannot help but wonder if it might have been a better idea to sell more drug at a lower price rather than the decision that was in fact made: price it sky high (on the cusp of a huge economic downturn and record low tax receipts for states, no less) and be forced to suffer the sluggish indignations of form filling and administrative sign offs in order to get it paid for. Even pharma circuiteer Dr. Robert Murphy conceded recently that a full half of the patients who actually want to take the drug (and even might continue to take after the first couple of months!) simply cannot get it.
During the course of an early February conference call, Trimeris CEO Dani Bolognesi outlined Trimeris’ plan to rescue their salvage therapy. “During the initial stage of this launch we have learned a great deal about the issues facing this unique drug and look forward to seeing the results of our heightened marketing initiatives on the uptake of Fuzeon in 2004,” he conceded to a group of investment types on February 3rd. Overall, the plan calls for huge increases in sales and marketing expenses — costs which certainly could not have been anticipated just six months ago. They include the following:
- Firing ChroniMed and establishing a multi-vendor distribution network.
- “Heightening awareness” of Fuzeon through “compelling” advertising and promotional campaigns. These print and Internet campaigns directed to patients, clinicians, pharmacists, nurses, and treatment educators will be implemented across the United States throughout 2004 and are “expected to continue in various forms beyond that time.”
- Dispatching bands of Fuzeon-trained nurses to the 20 or so principal Roche sales regions across the country in order to try to turn the tide of early terminators. The Fuzeon brigade will travel to physicians’ offices and patients’ homes to assist in the administration of the drug. (One can imagine the Cuckoo’s Nest scenario: “By god, you’re gonna take this drug even if I have to tie you down and inject it myself! And while we’re at it, maybe we’ll call in a few refill requests.”)[For the record, AAHIVM head and leading New York AIDS doc Howard Grossman cautions against lampooning the visiting nurse initiative. “It’s not about tying patients down,” he argued. “We’ve seen a lot of patients come through for studies, referred by other docs, who never got good teaching on injections. A bunch of them were handed the video and told to watch it and then do it. Of course, they’re having problems, increase injection site reactions, et cetera and quickly going off the drug. We’re finding that re-teaching people has some real benefit.”]
- Setting up so-called “out-bound” call centers. Basically, a telemarketing squad (think “Indirectly Observed Therapy” [IdiOT]) assigned the task of personally following up with all current, new and waffling Fuzeon patients (upon their assent) in order to assure that doses are not missed and that prescription refills are not delayed.
- “Upgrading” the Fuzeon patient mix. Put simply, Roche would like to move Fuzeon one or two steps up the treatment chain: so that docs would use it as part of a second- or third-line regimen — and not wait until a salvage or deep salvage situation. S.G. Cowen & Co. biotech analyst Yaron Werber, in a 26-page September report, observed that, “A key factor in Fuzeon’s prospective growth will be Trimeris/Roche’s ability to move beyond usage solely in deep salvage patients — [but instead] to include those triple-class experienced patients most likely to benefit from Fuzeon therapy.”
As of last September, Werber explained, physicians characterized 91% of Fuzeon patients as “deep salvage” and only 9% or so as second to third liners. But by March 2004, physician consultants to Trimeris assured them that the proportion of triple-class experienced (second and third liners) patients would increase to 35% — and those in deep salvage would represent the remaining 65%, thereby greatly increasing the overall market for the drug.” All available evidence, however, suggests that this is not in fact occurring.
In a way, just such a change in Fuzeon patient “mix” would probably more closely mirror the vaunted TORO study population. The problem with this strategy, of course, is that HIV-positive people who are leading relatively normal, active, mobile (pain free?) lives are unlikely to agree to the quality of life constraints twice daily Fuzeon administration entails. It’s the paradox of the entire Fuzeon program popping up, again and again: those who are most likely to benefit from the drug are the least likely to take it. Trimeris/Roche have at least three post-marketing clinical trial designs in the works. The highest priority for them (ready to go this spring, they say) will be the trial which attempts to strengthen the case for their new strategic positioning.
All this and a little sleight of hand (the company has expressed its wish to switch from counting actual patients on treatment to counting Fuzeon kits shipped — an accounting artifice which tends to pad the otherwise conservative IMS Health sales figures) may succeed in reversing the current downward business trajectory. But the key issues — price and formulation — do not appear to be part of the Fuzeon rescue plan.
Looming in the distance is a twice-spurned Roche family that could at any time decide to cut its losses and run. Partnership deals are apparently renewed one year at a time, and Roche has made its carrying of a large part of Fuzeon’s sales and marketing costs (all but $10 million of the $66 million for basically nine months of 2003 — nearly half that in the fourth quarter alone) contingent upon certain undisclosed “sales milestones” — an arrangement not uncommon in this kind of agreement. Sales and marketing expenses for 2004 are expected to continue to expand (~$80-90M), as Roche launches a re-election style marketing blitz, in addition to the outbound call centers and nomadic nursing teams. (Get a load of the latest Poz supplement: “Take Your Best Shot.”)
If Dr. Bolognesi is worried, he certainly didn’t show it on the February conference call. Every setback on the analysts’ laundry list of problems and disappointments was greeted enthusiastically by Bolognesi as yet another “opportunity.” And when cross examined by a very much engaged Yaron Werber about Roche’s near-term commitment to this product, Dani was similarly unfazed: “Roche continues to express their commitment to this product. They believe that Fuzeon will establish its rightful place in the treatment of experienced patients as we’ve said here today. They believe strongly that they can overcome the issues that are out there in the marketplace in the early phases of this launch. These guys are in it for the long haul.” We wish them well.
