By Joelle Dountio Ofimboudem
Malaria is a life-threatening disease prevalent in the world’s poorest countries, particularly in sub-Saharan Africa. Preventing malaria hits close to home for me. I am a mother to two children, aged three and five. We are originally from Cameroon, where we all have contracted malaria multiple times. As an intellectual property lawyer and global public health advocate, I know that geography — the fact that we live in the “neglected” part of the world in terms of research and development (R&D) priority — is the main reason for the constant battle with malaria. It is appalling to know that the multiple trips to the hospital and hospitalizations resulting in my kids missing school or not playing with their friends can be avoided.
Today, 14 of the 15 countries — which account for 80% of the malaria burden — are in sub-Saharan Africa. Yet, elimination efforts do not focus on these countries.1 While malaria remains a leading cause of death in sub-Saharan Africa, the disease was eradicated in the United States and Europe in 1951 and 1975 respectively.2 This is a stark reminder of the inequities in global health.
Malaria is a common comorbidity for people living with HIV. HIV infection can increase the risk and the severity of malaria, and the prevalence of symptomatic malaria is high among people who are coinfected with HIV.3 Moreover, malaria increases HIV plasma viral load and decreases CD4+ T cells,4 and both diseases cause complex activation of immune cells and disregulated production of cytokines and antibodies.5 Coinfection can also accentuate the progression of anemia.
As we explore long-acting technologies (LATs) for preventing malaria and HIV, many of the communities with whom we work are dramatically impacted by both and face similar access and linkage to care barriers (see Johnson). As a partner on the LONGEVITY project, which aims to develop LATs for preventing malaria and tuberculosis and curing hepatitis C, Treatment Action Group (TAG) applies our technical and community engagement expertise to follow and translate the science of LATs to benefit patients, affected communities, providers, researchers, and other stakeholders across these diseases.
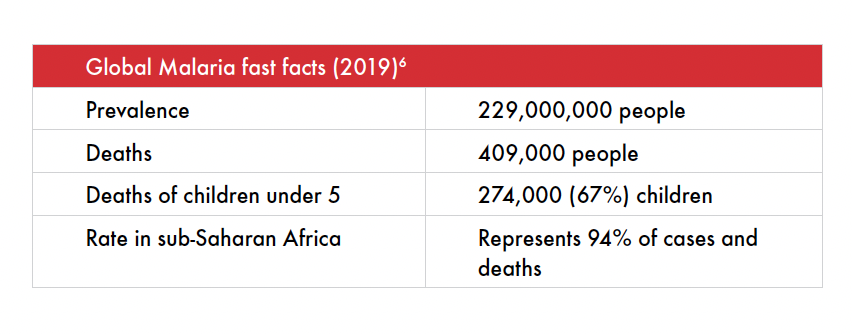 Current Malaria Prevention Measures and Challenges
Current Malaria Prevention Measures and Challenges
Malaria persists because current prevention measures do not go far enough, hence, the need for more comprehensive approaches in the battle against malaria. Currently, prevention revolves around three main interventions: (1) sleeping under insecticide-treated nets; (2) spraying insecticides indoors; and (3) providing malaria prevention medications to people traveling to malaria endemic countries, pregnant individuals, and infants living in high-transmission areas.7 Yet, each of these approaches presents challenges. People do not always use insecticide-treated nets because they increase heat,8 reduce airflow and cause breathing difficulties.9 The chemicals on these nets sometimes cause skin reactions; and the nets can be inconvenient for daily set-up in homes that lack space.10 Moreover, these nets are not typically replaced or re-treated in time to provide continuous protection against mosquito bites. With respect to indoor spraying, Pyrethroid — a chemical contained both in insecticide spray and insecticide-treated nets that kill mosquitoes— although affordable and having a long residual action, has developed resistance across most of malaria endemic sub-Saharan Africa due to increased use.11 The high cost of alternative insecticide sprays and their low community acceptance have also contributed to minimal spraying of insecticides indoors across sub-Saharan Africa.12 Lastly, insufficient capacity and funding for malaria prevention constrains countries’ ability to set their own agendas for reducing the disease burden.13
Current Malaria Treatment and Challenges
While there are several approved Artemisinin-based Combination Therapies for malaria treatment which are recommended by the World Health Organization,14 malaria treatment also faces many challenges Historically, due to high malaria prevalence in endemic countries such as Tanzania, health providers treated people with suspected cases of the disease with relatively cheap antimalarial medications, a practice known as presumptive treatment.15
In Cameroon, due to insufficient health staff, people stock and self-administer anti-malarial medications at the onset of any fever, especially in remote areas. The failure of authorities to effectively regulate medicines imports and quality floods the market with unverified/low quality antimalarials. Ineffective regulation provides patients with antimalarials through street vendors and even at pharmacies without prescriptions. There are also high costs: transportation, hospital consultation, and laboratory exams which, combined, cost more than the full treatment course for malaria from street vendors.
Due to these factors, and the frequency of symptoms, adults and parents self-administering malaria medication when they, or their children, have a fever is a common practice, despite healthcare guidelines and providers’ dissuasion. They often use unverified, low quality antimalarials that fuel drug resistance, or seek traditional herbal treatments at the onset of symptoms, and present too late at healthcare facilities with severe illness.
The LONGEVITY Project and Malaria Elimination
A long-acting malaria preventive — if proven safe, effective, acceptable, and accessible — could address many of the shortcomings of the current approaches to both treatment and prevention. By offering an easier way to protect people living in endemic regions against malaria, a long-acting formulation for malaria prevention would indirectly address drug resistance by sparing people from taking medications and eventually eliminating the market for unverified antimalarials.
Given the shortage of healthcare staff in most sub-Saharan African countries, a long-acting preventive for malaria would significantly reduce the workload on health providers and enable them to focus on other health problems — hence, enhancing public health overall. A long-acting malaria prevention therapy would also drastically reduce adult and infant mortality. More children could live more fulfilling and healthy lives, and parents like myself and their children would no longer have the burden of frequent visits to the hospital and hospitalizations. Apart from the health benefits, malaria elimination would reduce economic loss in the form of disability-adjusted life years in endemic countries.16
A long-acting formulation will hopefully eliminate the need for insecticide-treated nets, which cause skin reactions and exacerbate the already warm, tropical climates where malaria is endemic. People could sleep better, enhancing their overall well-being.
Malaria endemic countries usually have very fragile healthcare systems. Malaria prevention could help avert complications that arise with the onset of malaria, including anemia, miscarriage, liver failure, kidney failure, premature and stillbirth, and cerebral malaria. Given the high out-of-pocket cost, and the limited number of specialists available to provide care for these complications, especially in rural areas, prevention would be a tremendous public health win for malaria endemic countries.
The LONGEVITY Project – Redefining Pharmaceutical Research and Development
The pharmaceutical industry’s status quo has failed to develop new antimalarials and antibiotics because these medications have a lower return on investment than treatments for conditions that turn higher profits in high-income countries, such as cancer.17 The access-to-medicines movement has called for “push,” “pull,” and “pool” mechanisms to incentivize pharmaceutical developers and donors to invest in R&D for neglected diseases, such as malaria. The LONGEVITY Project encapsulates both the “push” (i.e., incentivizes industry by reducing R&D costs and absorbs upfront risks) and “pull” (i.e., creates incentives for private sector engagement by creating viable market demand) mechanisms. The project invests in R&D for long-acting injectables of existing treatments; in the case of malaria, it investigates atovaquone. The LONGEVITY Project also involves community engagement and advocacy to ensure accessibility and acceptability of long-acting formulations and prepare stakeholders for eventual introduction.
The high prevalence of malaria in LMICs over 50 years after its eradication in high-income countries is a glaring example of global health inequity. Malaria eradication in low- and middle-income countries would ensure a “malaria-free” world for all and enhance global health equity. Long-acting technologies offer a critical opportunity for advancing that goal.
Endnotes
1 Ranson H. Malaria: key challenges and potential solutions. High-Quality Technical Assistance for Results [Internet]. 2018 Apr 20 [cited 2021 Sept 6]; [about 11 a.].
https://www.heart-resources.org/reading_pack/malaria-key-challenges-potential-solutions/
[2] Thomson Reuters Foundation. Timeline: Seven decades of efforts to eradicate malaria. Reuters [Internet]. 2016 Oct 31 [cited 2021 July 20]. https://www.reuters.com/article/us-health-malaria-timeline/timeline-seven-decades-of-efforts-to-eradicate-malaria-idUSKBN12W2TS.3 Hochman S, Kim K. The impact of HIV and malaria coinfection: what is known and suggested venues for further study. Interdiscip Perspect Infect. 2009 Aug 9:617954. doi: 10.1155/2009/617954.
4 Alemu A, Shiferaw Y, Addis Z, Mathewos B, Birhan, W. Effect of malaria on HIV/AIDS transmission and progression. Parasites Vectors. 2013 Jan 17;6:18. doi: 10.1186/1756-3305-6-18
5 Ibid.
6 World Health Organization. Guidelines for Malaria. Geneva (CH): World Health Organization. 2021 July 13. pp. 23-70. https://www.who.int/publications/i/item/guidelines-for-malaria.
7 Ahorlu CS, Adongo P, Koenker H, et al. Understanding the gap between access and use: a qualitative study on barriers and facilitators to insecticide-treated net use in Ghana. Malar J. 2019 Dec 12;18(1):417. doi: 10.1186/s12936-019-3051-0.
8 Ngwibete A, Ozoms J. Attitudes toward utilization of insecticide-treated bed nets among pregnant women and care-takers of under-five. InfectionControl.tips [Internet]. 2016 Aug 14 [cited 2021 July 20]. https://infectioncontrol.tips/2016/08/14/insecticide-treated-bed-nets-731/
9 Ngwibete A, Ozoms J. Attitudes toward utilization of insecticide-treated bed nets among pregnant women and care-takers of under-five. InfectionControl.tips [Internet]. 2016 Aug 14 [cited 2021 July 20]. https://infectioncontrol.tips/2016/08/14/insecticide-treated-bed-nets-731/
10 Ibid.
11 Oxborough RM. Trends in US President’s Malaria Initiative-funded indoor residual spray coverage and insecticide choice in sub-Saharan Africa (2008-2015): urgent need for affordable long-lasting insecticides. Malaria Journal. [Internet]. 2016 March 8 [Cited 2021 July 20]; 15:146. Available from https://malariajournal.biomedcentral.com/articles/10.1186/s12936-016-1201-1.
12 Namuganga JF, Epstein A, Nankabirwal JI, et al. The impact of stopping and starting indoor residual spraying on malaria burden in Uganda. Nat Commun. 2021 May 11;12:2635. doi: 10.1038/s41467-021-22896-5.
13 Ranson H. Malaria: key challenges and potential solutions.
14 World Health Organization. Guidelines for Malaria. pp. 65-72.
15 Reyburn H, Mbatia R, Drakeley C, et al. Overdiagnosis of malaria in patients with severe febrile illness in Tanzania: a prospective study. BMJ. 2004 Nov 12;329(7476):1212. doi: 10.1136/bmj.38251.658229.55. Phillips V, Njau J, Li S, Kachur P. Simulations show diagnostic testing for malaria in young African children can be cost-saving or cost-effective. Health Aff (Millwood). 2015;34(7):1196–203. doi: 10.1377/hlthaff.2015.0095.
16 World Health Organization Regional Office for Africa. A Heavy Burden: The indirect cost of illness in Africa. Republic of Congo: World Health Organization. 2019. https://www.afro.who.int/publications/heavy-burden-productivity-cost-illness-africa
17 Plackett B. No money for new drugs. Nature Antimicrobial Resistance Outlook [Internet]. 2020 Oct 22 [cited 2021 July 20]; 586. https://media.nature.com/original/magazine-assets/d41586-020-02884-3/d41586-020-02884-3.pdf.
