Download:

By Erica Lessem and Lauren Volpert
September 2014
I. Introduction
Increasingly drug-resistant forms of tuberculosis (TB) are becoming more common worldwide, and few medicines are available to treat them.1 Newly developed TB drugs, such as bedaquiline and delamanid, offer some hope, but need to be taken along with other drugs. Linezolid, an antibiotic approved for the treatment of other bacterial infections—but not approved by any regulatory authority for the treatment of TB—can be an important drug in a regimen to treat drug-resistant TB when few other options exist. This guide highlights important information about linezolid’s safety and efficacy, and offers advocacy recommendations for activists, including community representatives, advocates, clinicians, researchers, policy makers, donors, and private-sector employees.
II. Mechanism of Linezolid
Linezolid kills TB by preventing the bacteria from turning their genetic information into active proteins (a process known as translation). Linezolid blocks the initiation step of translation so that the TB bacteria can’t make the proteins they need to function.2
Key Definitions and Acronyms
TB: tuberculosis
MDR-TB: multidrugresistant TB; or TB resistant to at least isoniazid and rifampin, the two most powerful existing TB drugs, which are used as part of the four-drug first-line therapy
Pre-XDR-TB: pre‑extensively drug-resistant TB; or MDR-TB that is resistant to either a secondline injectable drug (amikacin, kanamycin, or capreomycin) or a fluoroquinolone
XDR-TB: extensively drug-resistant TB; or MDRTB that is also resistant to a fluoroquinolone and at least one injectable second-line drug
III. Efficacy of Linezolid
Linezolid has not been tested in large clinical trials of people with TB, but information from observational studies and small trials supports the use of linezolid in highly resistant TB strains and cases that are otherwise complicated to treat (e.g., when someone with MDR-TB cannot tolerate other drugs in his or her regimen). A meta-analysis of 12 nonrandomized studies of linezolid’s role in MDR- and XDR-TB treatment found that 82 percent of patients treated with linezolid were cured or completed treatment—higher than in previously reported XDR-TB treatment outcomes.3
In one small trial, 41 people with XDR-TB on individualized regimens were randomized to receive 600 mg daily of linezolid, either immediately or after two months. After four months, or until culture conversion, they received either 600 mg or 300 mg of linezolid daily for at least an additional 18 months. While final results regarding linezolid’s effect on cure rates are pending, early data show that immediately starting linezolid increased the percentage of patients whose TB converted after four months (79% vs. 35%; P = .001), and that 87 percent of the 38 patients who received linezolid had a negative sputum culture within six months after starting linezolid.4 Only four patients developed linezolid resistance, despite having few other potent drugs in their regimen to protect against resistance.5 Another small study found linezolid in combination to be an effective option for children with drug-resistant TB, even when other treatments had failed.6
IV. Safety of Linezolid
Side effects and management All medicines have side effects, and though linezolid’s can be serious, they are clinically manageable. A systematic review found that more than half of patients receiving linezolid experienced side effects, and one-third of patients had to discontinue linezolid (see figure 2). Linezolid’s most common side effects are:
- optic and peripheral neuropathy (damage to the nerves in the eye and body), which can cause pain, numbness, weakness, and vision problems, and may be irreversible;7
- bone marrow suppression, resulting in low levels of red blood cells (anemia) or platelets (thrombocytopenia), which can lead to many health problems; and 8,9
- gastrointestinal disorders (discomfort or problems with the stomach, intestines, and esophagus).10
TB programs and doctors can monitor for and manage these side effects as part of routine care. Reducing the dose or changing the dosing schedule of linezolid may help: lowering doses to 600 mg per day or less may reduce side effects without making the drug less effective.11,12
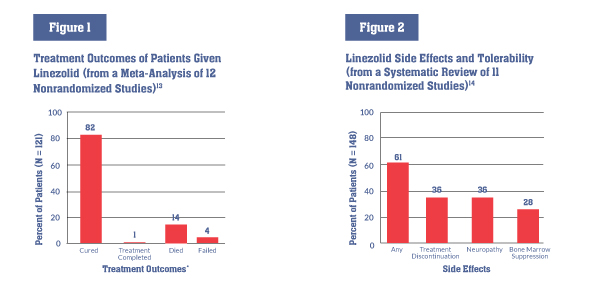
*Numbers do not add up to 100% due to rounding
V. Access to Linezolid
Limited availability and high prices can block access to linezolid for patients in urgent need. Linezolid was developed by Pfizer, which prices the drug exorbitantly (for example, about US$65 in South Africa—or US$46,800 for a 24-month treatment course at 600 mg per day—and US$154 in the United States per 600 mg pill—or US$110,800 for a 24-month treatment course).15,16 Cheaper, quality-assured generic versions are manufactured, however, such as a product by Hetero, which is available through the Global Drug Facility (GDF) for just US$6.9 per pill (US$4,968 for a 24-month treatment course).17 But access to cheaper options is not always possible in countries where Pfizer holds patents on linezolid; the primary patent expires in many countries in 2015, while secondary patents expire several years later.18 A lack of clear guidance from the World Health Organization (WHO) on the importance of linezolid is a barrier to access. Because there have been no large clinical trials of linezolid for TB, the WHO currently classifies linezolid as a “group 5” TB drug with an unclear role in the treatment of MDR-TB.19 Linezolid is also not on the WHO’s Essential Medicines List, which helps countries plan which drugs are necessary to treat important diseases such as TB.20 National TB programs have done little to prioritize the diagnosis and treatment of drug-resistant TB, and as such have not demanded linezolid. With recent efforts to diagnose more drug-resistant TB, and the approval of new treatments, bedaquiline and delamanid, demand for important companion drugs such as linezolid should increase.
Spotlight on approaches to access linezolid: South Africa and Moldova
In South Africa, Pfizer’s patent ownership and its refusal to lower prices have kept the price of linezolid at ZAR715 (US$65)21 per tablet in the private sector. In the public sector, the Pfizer product is purchased through the national antibiotics tender, but often not prescribed to DR-TB patients based on its high cost. Médecins Sans Frontières (MSF) and advocates’ attempts to expand access to linezolid recently accomplished a significant victory on June 26, 2014, when the South African Medicines Control Council (MCC) granted MSF special permission to use a generic linezolid product to expand access to DR-TB patients in its Khayelitsha project. MSF purchases the generic at US$8 per tablet—an 88 percent savings over the Pfizer product. The MCC approval for MSF paves the way for other health care providers—including the National Department of Health—to request similar permission to use generic linezolid within DR-TB treatment regimens. The decision could also set a precedent that facilitates full registration by the MCC of a generic linezolid product. Either action has the potential to expand access to the drug to hundreds more DR-TB patients in need across South Africa; currently, only MSF patients can benefit from this advance.
In June 2013, as part of Moldova’s efforts to stop XDR-TB, the national TB program and civil society representatives prioritized the procurement of linezolid. Moldova worked with the Global Fund to Fight AIDS, Tuberculosis and Malaria (GFATM) and was able to redirect savings from its GFATM grant toward the purchase of linezolid from the GDF. At the same time, it got permission from the national drug regulatory authority to import Hetero’s linezolid, even though it was not yet registered in Moldova, as the generic linezolid had a quality certificate. After months of bureaucratic negotiations, linezolid arrived in Moldova on January 27, 2014. The program now must ensure that patients are started on linezolid and continue to receive appropriate care.
VI. Take Action: Advocacy Messages
1. Linezolid is effective in the treatment of highly drug-resistant strains of TB.
While data supporting the efficacy of linezolid are limited, the drug’s importance in regimens for highly resistant strains is clear. In light of this:
- the WHO must publish guidance on the appropriate and responsible use of linezolid for treating MDR- and XDR-TB;
- the WHO should reevaluate its classification of linezolid as a “group 5” drug with an unclear role in MDR-TB treatment;
- the WHO should include linezolid on its Essential Medicines List (when it is updated in early 2015) in order to facilitate access;
- countries should include linezolid on their Essential Medicines Lists for TB; and
- national health departments must prioritize diagnosing and treating drug-resistant TB, and should include linezolid in their treatment guidelines.
2. High-quality research on linezolid for TB is needed.
Linezolid’s serious and frequent side effects mean that, currently, it is best suited for people with limited treatment options. More research is needed in both adults and children to determine whether linezolid can still be effective even at lower doses or with less frequent dosing, and if shorter treatment can reduce side effects. Research into potential new drugs that are in the same class as linezolid (such as AZD5847 and sutezolid), but that may be safer, is also needed. To this end:
- Pfizer (which closed its anti-infective unit in 2013) and public funders must support further research into linezolid for TB, including a large, well-conducted, randomized controlled trial;
- Pfizer and public funders should conduct additional studies to learn the correct dosage, timing, and duration of treatment to minimize side effects; to see if linezolid is safe for people with HIV taking antiretrovirals; and to know how best to safely and effectively combine linezolid with other new and existing drugs for TB;
- AstraZeneca (which also shut its TB research unit but claims that it will continue to invest in AZD5847) and Sequella (which took over the development of sutezolid from Pfizer) must adequately fund and prioritize research into new drug candidates that are in the same class of drugs as linezolid and may prove safer than linezolid; and
- AstraZeneca and Sequella should also collaborate with public and nonprofit TB drug trial sponsors to develop these drugs in effective combinations.
3. Registration of linezolid for a TB indication, the use of generics, appropriate pricing, and widespread procurement are necessary to ensure access to linezolid.
While research is pending, many people with TB urgently need linezolid, and we must work to ensure that they get it. To ensure access to linezolid:
- TB programs must procure linezolid: this includes budgeting for, buying, and distributing linezolid from quality-assured sources, preferably through the GDF;
- donors such as the GFATM must be flexible and support countries in planning for and funding this procurement;
- Pfizer (or a generic drug company) must prepare and submit a dossier to a stringent regulatory authority (that of Canada, European Union member states, the United States, or Japan) for linezolid to have an indication for use in the treatment of TB;
- Pfizer should agree not to assert its patent rights in countries where its secondary patents on linezolid would prevent procurement from cheaper, generic sources; and
- national drug authorities should permit the importation of quality-assured generic sources of linezolid, even when these are not yet registered in countries, given the urgency of treating drug-resistant TB.
Endnotes
- McKenna L, Zhang A, Lessem E. An activist’s guide to tuberculosis drugs. New York: Treatment Action Group; 2014. Available from: https://www.treatmentactiongroup.org/tb/drug-guide. (Accessed 2014 July 18)
- Swaney SM, Aoki H, Ganoza MC, Shinabarger DL. The oxazolidinone linezolid inhibits initiation of protein synthesis in bacteria. Antimicrob Agents Chemother. 1998 Dec 1;42(12)3251–5. Available from: http://aac.asm.org/content/42/12/3251.full.pdf. (Accessed 2012 May 15)
- Sotgiu G, Centis R, D’Ambrosio L, et al. Efficacy, safety and tolerability of linezolid containing regimens in treating MDR-TB and XDR-TB: systematic review and meta-analysis. Eur Respir J. 2012;40(6):1430–1442. doi: 10.1183/09031936.00022912.
- Lee M, Lee J, Carroll M, et al. Linezolid for treatment of chronic extensively drug-resistant tuberculosis. N Engl J Med. 2012;367(16):1508–1518. doi: 10.1056/nejmoa1201964. Cox H, Ford N. Linezolid for the treatment of complicated drug-resistant tuberculosis: a systematic review and meta-analysis. Int J Tuberc Lung Dis. 2012 Apr;16(4):447–54. doi: 10.5588/ijtld.11.0451.
- Lee M, et al. Linezolid for drug-resistant tuberculosis.
- Rose P, Hallbauer U, Seddon J, Hesseling A, Schaaf H. Linezolid-containing regimens for the treatment of drug-resistant tuberculosis in South African children. Int J Tuberc Lung Dis. 2012;16(12):1588–1593. doi: 10.5588/ijtld.12.0322.
- Cox H, et al. Linezolid for complicated drug-resistant tuberculosis.
- Ibid.
- Sotgiu G, et al. Efficacy, safety and tolerability of linezolid.
- Ibid.
- Chang KC, Yew WW, Cheung SW, et al. Can intermittent dosing optimize prolonged linezolid treatment of difficult multidrug-resistant tuberculosis? Antimicrob Agents Chemother. 2013 Jul;57(7):3445–3449. doi: 10.1128/AAC.00388–13.
- Sotgiu G, et al. Efficacy, safety and tolerability of linezolid.
- Ibid.
- Cox H, et al. Linezolid for complicated drug-resistant tuberculosis.
- Merck Manual. Linezolid: drug information. 2014 May. Available from: http://www.merckmanuals.com/professional/lexicomp/linezolid.html. (Accessed 2014 July 14)
- Médecins Sans Frontières. Linezolid fact sheet. 2014 July. Available from: http://www.msfaccess.org/content/linezolid-fact-sheet-0. (Accessed 2014 July 14)
- Stop TB Partnership. Linezolid product information. 2014. Available from: http://www.stoptb.org/gdf/drugsupply/pc3.asp?PID=818. (Accessed 2014 July 14)
- Drugs.com [Internet]. Generic Zyvox availability. September 23, 2014 [cited 2014 July 14]. Available from: http://www.drugs.com/availability/genericzyvox.html.
- McKenna L, et al. An activist’s guide to tuberculosis drugs.
- World Health Organization. WHO model lists of essential medicines. 2013. Available from: http://www.who.int/medicines/publications/essentialmedicines/en. (Accessed 2014 July 16)
- We converted data reported in non–U.S. currency into U.S. dollars using the September 12, 2014, currency exchange rate provided by the OANDA Corporation: http://www.oanda.com/currency/converter.
