July 2012
By Simon Collins
NOTE: a postscript section at the end of this article includes periodic updates since publication in June 2012.
Updates from CROI 2013. (March 2013) at the end of this article.
INTRODUCTION
Two aspects of antiretroviral treatment over the last year have developed along separate paths despite their clear connection. The degree to which they are tied is easier to speculate on than to predict, and this has a new significance for HIV pipeline research.
The first—and the traditional focus for this annual pipeline review—is the mainstream development of new compounds through early and regulatory phases of development, hopefully to approval and postmarketing research.
The development pyramid rising from tens of thousands of potential molecules screened to achieve one marketed drug is well described. This report summarizes the progress of compounds that generally have results from phase II/III studies, and this year it highlights a new dynamic for the coformulated end product to become elevated, in many cases, above that of an individual new drug.
So within a couple of years, there might be half a dozen single-pill, once-daily, fixed-dose combinations (FDCs). For a doctor to be able to say, “Which one of these six pills would you like to take each day?” is a significant achievement for anyone who remembers the complexities of early HAART (handfuls of pills with multiple doses and diet restrictions) ― even if, in the detail, virological failure with resistance to one FDC is likely to preclude subsequent reliance on others. Intercompany collaborations are unusual in other health areas, but traditional obstacles have been overcome by some companies for HIV formulations. The Western market for better drugs is still both highly competitive and lucrative.
However, the second strand of development, progressing just as insistently, is the funding pressure on health services, especially in countries where they are based on public health– or donor aid. During an economic recession, in both rich and poor countries, the potential impact of patent expiration on commonly used, established drugs challenges research-based companies to not only produce better, more effective, and safer drugs, but then bring them to market at a price that will enable them to be widely used.
These economic pressures already focus the concerns of health workers and activists as much as those of health care purchasers. For most people with access to treatment, HIV has become a largely manageable illness. As such, it now has to work within mainstream health budgets. When resources are limited—and by definition they always will be—the priority for access to funding must be determined by some comparative evaluation of need. Until now, the price for new drugs generally incorporated some mark-up for added value, but antiretroviral (ARV) combinations for first-line therapy have broadly operated in a similar ballpark of approximately US$10,000 per year.
The potential for generic versions of widely used drugs to undercut drug costs in developed countries may drive the need for similarly competitive pricing for newly approved drugs, at least for countries whose health care systems have the least flexibility for premium pricing. Most insurance-based sectors of the United States may be protected, but in June 2012, the AIDS Drug Assistance Program (ADAP) still had more than 2,000 people on its ARV waiting list. The U.S. patent expiry on efavirenz in 2013 may change prescribing practice for the FDC Atripla, given its prominent role as a preferred first-line drug. The U.S. patent for nevirapine expired in November 2011, and by May 2012 the FDA had approved generic formulations from 10 different manufacturers.[1] The combined formulation of AZT/3TC is also now off patent, with generic versions available.
By contrast, the cost-effectiveness of treatment at today’s prices might also prompt generic companies to charge high prices, even with competition. When ddI, AZT, 3TC, and most recently nevirapine came off patent, even recognizing the more limited clinical uses, generic drug prices in Western countries were only modestly reduced compared to those of brand drugs. An indication of the importance of the financial constraints of public health care systems, however, is that even these relatively small savings have been sufficient for some countries to switch patients who were previously stable on coformulated medications such as Truvada (tenofovir + FTC) or Epzicom/Kivexa (abacavir + 3TC) to either tenofovir or abacavir, plus generic 3TC, based on little difference between 3TC and FTC. Other measures to reduce drug budgets, highlighted in the opening lecture for the pharmacology workshop this year, include pressing companies for greater discounts, discontinuing the most expensive drugs, using cheaper options preferentially, and using boosted-PI monotherapy—a strategy that was initially developed, at least in part, for U.S. patients with limited health care insurance.[2] In Europe, many of these measures are already being used in Portugal, Spain, and the United Kingdom.
Fortunately, the fiscal basis of insurance-based health care systems, especially in the United States, however problematic a model for public health care, remains sufficiently strong for industry analysts to still confidently predict that HIV drug development will continue to offer lucrative returns on investment, and that future uptake of higher priced new drugs will offset the impact of generics.[3]
For anyone following pharmaceutical PR, it also presents the unnerving spectacle of some companies highlighting inadequacies of their own established drugs to promote newer compounds that are developed based on noninferiority studies.
Summary of Progress of Pipeline Compounds
Developments for individual compounds over the last year are summarized in Table 1. These include both updates from last year’s report and data on new compounds that advanced from preclinical phases of development.
Each of the compounds is then discussed in more detail.
TABLE 1. Summary of Pipeline Compounds in 2012
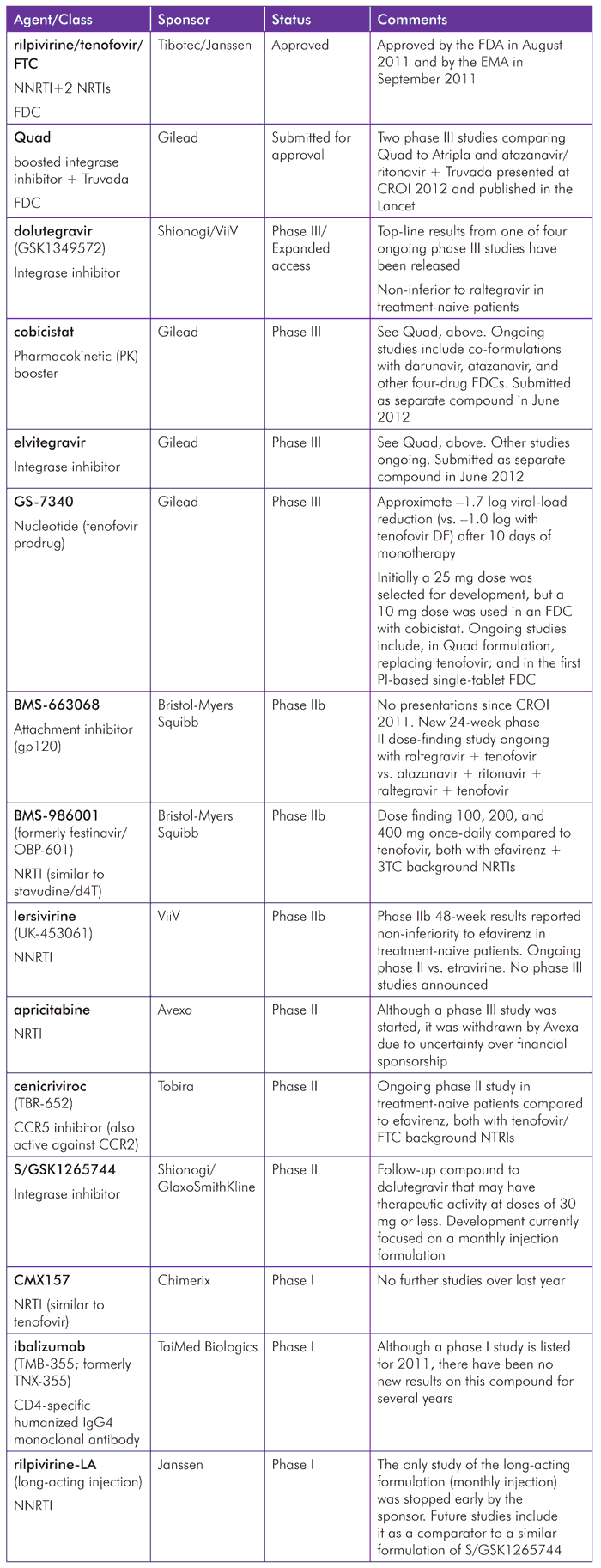
Approvals since the 2011 Report
As we went to press, only one new compound had been licensed since the last pipeline report. This was a fixed-dose combination (FDC) of the NNRTI rilpivirine coformulated with tenofovir and FTC that was approved by the U.S. Food and Drug Administration (FDA) in August 2011 (as Complera), and by the European Medicines Agency (EMA) in September 2011 (as Eviplera).[4]
Rilpivirine had already been approved in the United States a couple of months earlier. Notably, this received an indication only for the treatment-naive, with a caution to use it in patients with viral load <100,000 copies/mL and highlighting the importance of adherence, coadministration with food, and the potential for cross-resistance with both first- and second-line NNRTIs.[5]
However, several exciting compounds are on the brink of regulatory decisions, and others are in advanced phase III studies.
Update on Compounds with Phase II/III Results
The upcoming pipeline can be categorized broadly as “hopeful,” “early days,” and “trailing.”
- Hopeful: Quad (elvitegravir/cobicistat/tenofovir/FTC), elvitegravir, cobicistat, GS-7340, dolutegravir, 572-Trii (dolutegravir/abacavir/3TC), and Quad variations (using GS-7340 and darunavir).
- Early days: S/GSK1265744, lersivirine (UK-453061), BMS-986001 (formerly OBP-601), BMS-663068, cenicriviroc (TBR-652), rilpivirine-LA (long-acting injection).
-
Trailing: apricitabine, ibalizumab (TMB-355; formerly TNX-355), CMX157, CTP-518.
Quad: Elvitegravir/Cobicistat/Tenofovir/FTC
Currently in development by Gilead, Quad is a single-tablet FDC of elvitegravir (an integrase inhibitor), cobicistat (a pharmacokinetic [PK] booster), tenofovir (a nucleotide), and FTC (a nucleoside) that is taken once daily with food. Quad was submitted to the FDA in October 2011, with a decision expected by August 2012.
Top-line results from two randomized, double-blind, placebo-controlled phase III studies were released in October 2011, presented at the 19th Conference on Retroviruses and Opportunistic Infections (CROI) in March 2012, and published in the Lancet. They compared Quad to efavirenz/tenofovir/FTC (Atripla) in one, and to atazanavir/ritonavir plus tenofovir/FTC in the other.[6],[7]
In May 2011, the FDA Advisory Committee reviewing Quad voted 13–1 to recommended approval based on general safety and efficacy, but highlighted renal complications (the vote against was from a renal specialist, and based on lack of safety data compared to existing options). The limited data on use in women and African Americans, among whom renal disease is more prevalent, have also been noted by the FDA.[8]
The primary endpoint in both phase III studies was the proportion of patients with undetectable viral load (<50 copies/mL) at week 48 by intention-to-treat analysis, with noninferiority defined by a lower margin of –12%, and included patient stratification by baseline viral load above and below 100,000 copies/mL. Virological efficacy was around 90% (though median baseline viral load was only 31,000 copies/mL in one study), tolerability was good, and discontinuations were notably low in all arms. Both found Quad to be noninferior to the comparator combinations.
Study 236-0102 compared Quad to Atripla and enrolled 700 treatment-naive patients in the United States and Puerto Rico.[6]
Baseline characteristics included a mean age of 38 years and low median viral load (31,000 copies/mL), although one-third of participants started at >100,000 copies/mL. Mean CD4 count was just under 400 cells/mm3, with 12% of participants starting below 200, 32% starting at both 200–350 and 350–500, and 23% starting at >500 (percentages for Quad arm, but similar to Atripla). The study group was largely male (88%), with ethnicity 61% white, 31% African American, and 8% other. Fewer than 5% of participants in each arm had either HBV- or HCV coinfection.
Discontinuations before week 48 occurred in 11% versus 13% in the Quad versus Atripla arms for broadly similar reasons.
Viral load was suppressed to undetectable in 88% versus 84% of patients (difference +3.6%, 95%CI, –1.6 to +8.8) meeting criteria for noninferiority, with 7% of patients in each arm having virological failure, and 5% versus 9% having missing data (all Quad vs. Atripla, respectively). Responses by subgroup (viral load, CD4 count, race, sex, age, and adherence level) were not significantly different, but tended to favor Quad. CD4 increases favored the Quad arm, with +239 cells/mm3 versus +206 cells/mm3 respectively (P = 0.009).
Approximately half of the patients in each arm failed with mutations associated with resistance to either integrase inhibitors (mainly E92Q) or NNRTIs (mainly K103N) in 8 out of 14 versus 8 out of 17 patients, respectively.
Most side effects were reported as mild (grade 1), with statistically significant differences including more nausea in the Quad arm (21% vs. 14%), and more abnormal dreams (15% vs. 27%), insomnia (9% vs. 14%), dizziness (7% vs. 14%), and rash (6% vs. 12 %) in the Atripla arm.
Discontinuations related to side effects occurred due to rash (0% vs. 1.4%), renal abnormalities (1.4% vs. 0%), depression (0.3% vs. 0.9%), abnormal dream (0% vs. 0.6%) in the Quad versus Atripla arms, respectively, with 3% in each arm stopping due to each of fatigue and paranoia.
The most frequent grade 3 or 4 laboratory abnormalities occurring in more than five patients in each arm were broadly similar and generally low, including creatinine kinase (5% vs. 11%), AST (2% vs. 3%), ALT (1% vs. 3%), GGT (2% vs. 5%), neutrophils (2% vs. 3%), amylase (2% in each arm), and hematuria (2% vs. 1%), all in Quad versus Atripla, respectively.
Serum creatinine increased by approximately 0.1–0.2 mg/dL by week 2 in the Quad arm that was maintained through to week 48, compared to no change with Atripla (P < 0.001).
Increases in fasting total cholesterol (TC), LDL cholesterol, and HDL cholesterol were significantly greater in the Atripla compared to the Quad arms, but there was no difference among groups in the more clinically significant TC/HDL ratio or in triglycerides (+7 mg/dL in each arm).
The second Quad study, called 236-0103, compared Quad to atazanavir/ritonavir, a boosted HIV protease inhibitor, plus tenofovir/FTC (Truvada) in 708 treatment-naive patients.6 Baseline characteristics were broadly similar to the 236-0102 study: mean age 38 years, 90% male, and 74% white. CD4 count, viral load, and hepatitis coinfection were also similar, with 40% of participants having a viral load ≥100,000 copies/mL (but median was slightly higher at 63,000 copies/mL). Exclusion criteria for this study included renal function defined as eGFR <70 mL/min.
Virological efficacy (<50 copies/mL at week 48) was 92% versus 88% (difference +3.5%, 95%CI, –1.0% to +8.0%) in favor of Quad, which met the criteria for noninferiority. In patients with baseline viral load ≥100,000 copies/mL, response rates were 85% versus 82% (P = NS). Virological failure (FDA snapshot algorithm) was 5% in both arms. Median CD4 increases in this study were similar at +207 cells/mm3 versus +211 cells/mm3, and discontinuation rates for side effects were 4% versus 5% (in Quad and atazanavir/r arms, respectively).
Side effects occurring in ≥5% of patients were similar in each arm, apart from elevated bilirubin levels, which were significantly higher in the atazanavir/ritonavir arm. Discontinuations occurred due to diarrhea (4% vs. 5%); pyrexia (1% vs. <1%); nausea (1% vs. 0%); vomiting and fatigue (each <1% vs. 1%); and jaundice, dizziness, ocular icterus, and drug eruption (each 0% vs. <1%). The most frequent grade 3 or 4 laboratory abnormalities occurring in at least 2% in either arm were broadly similar, including creatine kinase (6% vs. 7%); hematuria (4% vs. 2%); AST (2% vs. 3%); ALT (2% vs. 2%); amylase (2% in each arm); and increased bilirubin (1% vs. 58%), all in Quad versus atazanavir/ritonavir arms, respectively. Serum creatinine increased by approximately 0.08 mg/dL by week 2 in the Quad arm, and was 0.12 mg/dL at week 48, compared to 0.05 mg/dL with atazanavir/ritonavir (P < 0.001). Median change in CLCr from baseline was –12.7 mL/min in Quad and –9.5 mL/min (P < 0.001) in the atazanavir/ritonavir arm. Lipid increases were similar for TC, LDL, and HDL cholesterol (all P = NS), but triglycerides increased by less in the Quad arm (+5 mg/dL vs. +23 mg/dL; P = 0.006).
Median changes in bone mineral density were similar in each group. Spine changes reduced by about 3% at week 24 and remained stable, with reductions at week 48 of –2.45% versus –3.48% (P = 0.25 for between-arm comparison). Reductions at the hip were continuous slopes for both combinations of about –1.5% versus 2.0% at week 24, and –2.87% versus 3.59% at week 48 (P = 0.12).
Safety data compiled for the 206-page FDA briefing document reported renal adverse events of 1.6% for Quad (N = 12) compared to 0.5% (N = 2) for Atripla and 0.6% for atazanavir (N = 2), leading to six discontinuations in the Quad group versus one with atazanavir. Two of these patients had eGFR <70 at baseline or screening. Rates for acquired Fanconi syndrome and renal tubular disorder were reported as 0.7% and 0.3%, respectively.[8]
These results all broadly support this important new FDC option. At least three phase III studies are already ongoing for patients currently stable on PIs, NNRTIs, or other integrase-based combinations to switch to Quad.[9],[10],[11]
Elvitegravir (GS-9137)
Elvitegravir is a once-daily integrase inhibitor that, with boosting (150 mg cobicistat or 100 mg ritonavir), has a plasma half-life of 9.5 hours, and achieves mean viral-load reductions of approximately 2 log copies/mL after 10 days of monotherapy.[12]
Elvitegravir is metabolized primarily by CYP3A and secondarily via UGT1A1/3, and requires a reduced dose (from 150 mg to 85 mg daily) if used in combination with atazanavir. Recent studies reported no interactions with rosuvastatin, but noted that rifabutin and other mycobacterial drugs are currently contraindicated.[13],[14]
Although it has already been submitted for regulatory approval as a component of Quad, and the majority of ongoing research includes Quad, elvitegravir is also included in a similar FDC from Gilead that uses the tenofovir prodrug GS-7340 in place of tenofovir.
Elvitegravir is also being developed as a separate compound, though the subject of more limited research.
A phase III randomized study is currently comparing elvitegravir/ritonavir to raltegravir (with both arms using additional drugs) in treatment-experienced patients. Now enrolled, the study should produce results shortly.[15]
Elvitegravir was submitted to the FDA as a separate compound in June 2012.[16]
Cobicistat (GS-9350)
Cobicistat is a PK booster that is a potent inhibitor of cytochrome P450 3A4. It is a weak inhibitor of 2D6 but not other CYP or UGT pathways, and has an inhibitory effect similar to ritonavir’s on other transporters that affect drug metabolism, including Pgp, BCRP, and OATP1B1/3.[13],[17]
Cobicistat also appears to have a short-term side-effect profile similar to that of ritonavir, including gastrointestinal and lipid effects, but without antiviral activity. Although the potential for renal complications has been raised in studies based on its use in Quad, mean eGFRs at week 24 were stable, and similar to ritonavir in a phase II study (that included tenofovir). The strategic importance of cobicistat is as a new option to develop advances in boosted formulations.[18]
Compiled renal data prepared for the Quad submission to the FDA reported similar renal events in the cobicistat versus ritonavir studies (N = 6 each), with a 1.5% discontinuation rate for cobicistat. Cobicistat also produces a small increase in serum creatinine that results in a small decrease in estimated but not actual GFR. An outstanding issue related to clinical management of increases in serum creatinine seen in both Quad studies has been proposed: an increase of 0.4 mg/dL or greater may be able to be used as a conservative cut-off to address concerns about potential tenofovir renal tubular toxicity.[8],[19]
As with elvitegravir, the PK booster cobicistat has already been submitted for regulatory approval as a component of Quad. It is also coformulated in an FDC with elvitegravir, FTC, and the tenofovir prodrug GS-7340, and, in a collaboration between Gilead and Janssen, with an HIV protease inhibitor, darunavir, FTC, and GS-7340.[20],[21]
There are also plans for collaborations between Gilead and Janssen (for darunavir) and BMS (for atazanavir) to combine cobicistat with these PIs to eliminate the need for a separate booster.[22],[23]
Limited research is ongoing for cobicistat as a separate compound, but 48-week results are expected in 2012 from the phase II study comparing cobicistat to ritonavir as a booster for atazanavir in treatment-naive patients.[19]
Results from a recent study presented at the 13th International Pharmacology Workshop included data supporting the safety of using cobicistat twice daily (150 mg BID resulted in approximately fourfold higher exposure compared to 150 mg once daily). While the impact of cobicistat when boosting darunavir is similar to ritonavir, this is not seen with tipranavir (an HIV protease inhibitor used for multidrug resistance). Tipranavir exposure is markedly lower when boosted by cobicistat, and cobicistat exposure is 90% lower compared to cobicistat alone.[24]
Comparable bioavailability results were also presented for two fixed-dose formulations of darunavir/cobicistat (800 mg/150 mg) when compared with darunavir/ritonavir (800 mg/100 mg).[25]
Ongoing phase III studies use cobicistat in combinations that boost darunavir or atazanavir and include a safety study for patients with mild to moderate renal impairment at baseline.[26]
Cobicistat was submitted to the FDA as a separate compound in June 2012.[27]
GS-7340
GS-7340, in development by Gilead, is a prodrug of the NRTI tenofovir, but has higher potency at much lower concentrations, and will also use less active pharmaceutical ingredients (API) in relation to viral impact, something that affects the final cost significantly in resource-limited compared to Western settings. This is important given the reliance on stavudine which—despite its toxicities—is still used by perhaps 50% of people on treatment globally who have been unable to access tenofovir. The low milligram dose will also extend its use in co-formulations with other ARVs.
While tenofovir is extensively used—estimates suggest by perhaps 50% of patients in Western settings, and Gilead now markets based on “nine million patient years experience”—there remain concerns about its potential long-term impact on renal function. In 2012, two large cohort studies—the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) study in Europe, and a study at the U.S. Department of Veterans Affairs—both reported associations between tenofovir use and renal health in patients with normal renal function at baseline.[28],[29]
Compared to the current formulation of tenofovir, the in vitro median effective concentrations (EC50s) for GS-7340 are 0.008 uM versus 0.05 uM in MT-2 cells, 0.003 uM versus 0.015 uM in PBMCs, and 0.014 uM versus 0.06 uM in macrophages. An initial dose-finding 10-day monotherapy study with an early formulation reported viral-load reductions of about –1.0 log at 50 mg and 150 mg doses, compared to 0.5 log with TDF, with plasma concentrations of GS-7340 that were 88% lower, and intracellular concentrations fourfold higher, compared to TDF.[30]
At CROI 2012, a similar dose-finding study randomized 38 treatment-naive or -experienced (but tenofovir-sensitive) patients to 10 days GS-7340 monotherapy using 8 mg, 25 mg, and 40 mg of a new formulation, with placebo and TDF arms as controls. The primary endpoint was the time-weighed average change in viral load (DAVG) at day 11.[31]
Baseline characteristics included: age 38 years; 97% male; and 50% white/38% African American. The mean viral load and CD4 counts were 31,000 copies/mL and 478 cells/mm3, respectively.
DAVG results were –0.76, –0.94, –1.13, –0.48, and –0.01 log copies/mL in the 8 mg, 25 mg, 40 mg, TDF, and placebo arms, respectively, with median viral-load reductions of –1.08 log (8 mg), –1.46 log (25 mg), –1.73 log (40 mg), –0.97 log (TDF), and –0.07 log (placebo). There were significant differences between both the 25 mg and 40 mg arms when compared to TDF, but not for the 8 mg dose.
Plasma tenofovir exposures across the GS-7340 groups were approximately 80–97% lower compared to TDF, with intracellular concentrations in PBMCs sevenfold higher with the 25 mg dose, and twentyfold higher with the 40 mg dose.
There were no clinically significant laboratory abnormalities or drug-related serious adverse events, no discontinuations, and no evidence of resistance over the 10 days.
Although no renal concerns were seen after 10 days, this will be an important aspect of further studies, including whether increased intracellular concentrations of GS-7340 accumulate in renal tubule cells. In vitro data on MT-2 cells, PBMCs, and macrophages did not find increased levels of intracellular diphosphates. CNS penetration by GS-7340 is expected to be similar to TDF.
Although selection of the 25 mg dose for single compound has been reported, a pharmacokinetic interaction with cobicistat that boosts GS-7340 supported use of 10 mg doses in coformulations;[32] this includes with elvitegravir/cobicistat/FTC, and with darunavir/cobicistat/FTC in the first PI-based single-tablet FDC,14 both of which are currently in ongoing phase II studies.[33],[34] The interaction of renal complications with the renal impact of cobicistat will also be a key aspect of these studies.
Dolutegravir
Dolutegravir is an integrase inhibitor being developed by ViiV that has advantages over raltegravir and elvitegravir. It is dosed once daily in treatment-naive patients and twice daily in treatment-experienced patients; requires no boosting; and has low PK variability, a potentially distinct resistance profile to raltegravir, and high potency at a low milligram dose.[35],[36]
Dolutegravir is metabolized primarily by UGT1A1, using CYP3A as a minor route (10–15%), but it does not have a clinical impact of inducing or inhibiting major CYP, UGT, or transporter pathways (except OCT2). It is expected that interactions will be able to be clinically managed by dose adjustment, when appropriate. Currently known interactions include significantly increased dolutegravir exposure with atazanavir (boosted and unboosted), and reduced exposure with darunavir, fosamprenavir, tipranavir, efavirenz, and rifabutin (by 30–75%; not considered clinically significant for treatment-naive patients). However, etravirine reduces dolutegravir exposure by 88%, and can be used only if coadministered with lopinavir/r or darunavir/r (which increase dolutegravir exposure). Dolutegravir needs to be given twice-daily with rifampin and antacids separated by at least two hours (due to metal cation chelation rather than a pH effect).[37]
There are encouraging safety data out to 96 weeks from phase II studies,[38] and phase III results reported noninferior top-line results compared to raltegravir in treatment-naive patients.[39] In addition, a study with two FDC formulations of dolutegravir with abacavir and 3TC (compound name: 572-Trii) has been completed,[40] and encouraging results have already been presented for a pediatric sprinkle formulation.[41] Dolutegravir is already available in an expanded access program.[42],[43],[44]
In April 2012, results from the phase III SPRING-2 study comparing dolutegravir to raltegravir in treatment-naive patients reported noninferiority based on viral suppression (<50 copies/mL) in 88% versus 85% in the dolutegravir versus raltegravir arms, respectively (95%CI, –2.2% to +7.1%), with the lower margin of the 95% confidence interval being above the prespecified –10%. No tolerability differences were noted between arms.[45]
Results from the SPRING-1 dose-finding study of dolutegravir/abacavir/3TC compared to efavirenz/tenofovir/FTC (Atripla) in treatment-naive patients were presented in a late-breaker oral session at CROI in 2012, and were broadly similar at 96 weeks to 48-week results for the 50 mg arm.[38]
Two hundred and five participants were randomized to receive dolutegravir at 10 mg, 25 mg, or 50 mg once daily compared to efavirenz. Baseline demographics included: 86% were male; 80% were white; 26% had baseline viral load >100,000 copies/mL; and 67% used tenofovir/FTC as the NRTI backbone.
At week 96, the proportion of patients with viral load <50 copies/mL (TLOVR) was 79%, 78%, and 88% in the 10 mg, 25 mg, and 50 mg arms, respectively, versus 72% in the efavirenz arm. Virological failure occurred more frequently in the lower-dose arms: in 13% (N = 7), 8% (N = 4), 4% (N = 2), and 8% (N = 4) of the 10 mg, 25 mg, 50 mg, and efavirenz arms, respectively, but these were low study numbers, and half these patients who counted as treatment failures by TLOVR analysis resuppressed to <50 copies/mL by week 96. No mutations associated with resistance to integrase inhibitors or NNRTIs were seen in these patients.
CD4 count increases were not statistically different at week 96: +338 cells/mm3 for the combined dolutegravir arms versus +301 cells/mm3 for efavirenz (P = 0.155).
Only two people discontinued dolutegravir due to side effects (one in each of the 25 mg and 50 mg arms) compared to five in the efavirenz group. Side effects were lower in the dolutegravir arms, although serious side effects were similar. The only grade 3/4 lab abnormalities were single cases of ALT elevation associated with acute hepatitis C. No differences in renal markers were observed between the two groups.
There are ongoing phase III studies of dolutegravir in treatment-experienced patients with resistance to raltegravir or elvitegravir, and to darunavir/ritonavir in treatment-naive patients.[45],[46],[47],[48]
Results from research in treatment-experienced patients with mutations associated with virological failure after using other integrase inhibitors have been encouraging, after the adoption of an increased dose (to 50 mg twice daily), given the historical difficulties of within-class resistance.[49],[50]
The expanded access program for dolutegravir is already open in Europe and the United States, although with a caution on the importance of using it in combination with other active drugs in order to avoid early resistance and further loss of treatment options in patients with complicated multidrug resistance.[42],[43],[44]
S/GSK1265744
While the focus on dolutegravir generated considerable excitement, there is also interest (though minimal data) on the follow-up integrase compound at GSK/ViiV called S/GSK1265744, which has a longer half-life (approximately 30 hours vs. 15 hours for dolutegravir).
The early phase I/II results presented at the Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC) in 2009 reported a median viral-load reduction of 2.6 log copies/mL following 10-day monotherapy at a 30 mg once-daily dose in treatment-naive patients, with discussion that a lower milligram dose may also be possible.[51]
A long-lasting injection formulation is also being investigated to compare its pharmacological properties to those of both oral administration and the long-acting formulation of the NNRTI rilpivirine, with potential use as both treatment and preexposure prophylaxis (PrEP).[52],[53]
Update of Other Compounds
Lersivirine
Lersivirine (previously called UK-453061) is a once-daily NNRTI owned by ViiV that was originally developed by Pfizer, and that has a resistance pathway at V108I that appears distinct from the K103N or Y181C pathways associated with first-generation NNRTIs.
The latest data come from a phase II double-blind, placebo-controlled study that randomized 193 patients (1:1:1) to either 500 mg or 750 mg of lersivirine or to standard-dose efavirenz, each with once-daily tenofovir/FTC. The primary endpoint was the percentage of patients with viral load reduced to <50 copies/mL at 48 weeks, with follow-up out to 96 weeks (by ITT missing = failure analysis).[54]
Baseline CD4 count and viral load were median 310 cells/mm3 (range 122—955) and mean 50,000 copies/mL (range 1,500–1,600,000), respectively. Approximately 35% of patients had baseline viral load >100,000 copies/mL, and this was reflected in prespecified analysis of the results.
Other baseline characteristics included: mean age 36 years (range 21–62); 27% female; and 60% white/30% black/10% other. While the majority of people had subtype B, approximately 30% of people had subtype C related to patients at South African sites.
At week 48, the percentage of patients with viral load <50 copies/mL was 79%, 79%, and 86% in the 500 mg, 750 mg, and efavirenz groups, respectively. Although the study was not powered to detect a difference in efficacy among arms, the lersivirine arms suggested a poorer response compared to efavirenz (500 mg: –9% difference; 80%CI, –18.1, 0.8; and 750 mg: –8% difference; 80%CI, –17.0, 1.2).
Results stratified by baseline viral load (which was lower in the >100,000 copies/mL group) or geographical region (which was lower for sites in South Africa) did not contradict this finding. Mean CD4 count increased approximately +190 cells/mm3 from baseline and was similar.
Limited data on virological failure (in 4, 5, and 3 patients in the 500 mg, 750 mg, and efavirenz groups, respectively) indicated that the lersivirine arms were associated with M184V plus NNRTI mutations when resistance was isolated. The one person with identifiable mutations in the efavirenz arm failed with K103N alone.
Overall, the combined safety analysis reported a similar incidence of side effects in each group, but fewer grade 3/4 events in the lersivirine groups (N = 2 and 3) compared to efavirenz (N = 8). Laboratory abnormalities were infrequent and evenly distributed among arms. Lipids were broadly stable for lersivirine compared to increases in TC, LDL, HDL, and triglycerides (TG) for efavirenz, but this resulted in little difference between the lersivirine and efavirenz groups (+0.24 and –0.06 vs. –0.3) in the change in the TC:HDL ratio, which is used to evaluate cardiovascular risk.
However, the study concluded that both lersivirine doses showed similar efficacy to efavirenz over 48 weeks in treatment-naive patients and had different side effect profiles compared with efavirenz.
While there is still a role for a new NNRTI with activity against nevirapine- and efavirenz-associated resistance, the higher reports of nausea and headache, even if low grade, might explain why no further clinical research is ongoing other than follow-up of patients in the initial studies.[55]
BMS-986001 (NRTI)
BMS-986001 (previously OBP-601 and, briefly, festinavir) is an NRTI with a structure similar to that of stavudine (d4T), but a safety profile that is unlikely to be associated with similar side effects. In vitro studies suggest that BMS-988001 is a weak inhibitor of DNA synthesis, and unlikely to affect mitochondrial function.
The most recent data, first reported in 2010, come from a revised analysis of the phase I/II dose-finding study in treatment-experienced patients (off treatment for at least three months). Following 10 days of monotherapy, median reductions in viral load on day 11 were 0.97, 1.15, 1.28, and 1.15 log in the 100, 200, 300, and 600 mg groups, respectively (vs. –0.07 in the placebo group), from median baseline levels across groups of 4.3–4.6 log (range 3.5–5.3 for the whole study).[56]
In vitro data on the drug susceptibility of BMS-986001, including susceptibility to the Q151M NRTI multidrug-resistant mutation, were presented in a poster at CROI in 2008. Although antiviral activity was reduced in the presence of most viruses carrying nucleoside-associated mutations (5- to 10-fold), including M41L (0.3- to 4.3-fold) and D67N (1.6- to 7.8-fold) resistance mutations, together with K103N with or without M184V. Viruses carrying the Q151M mutation were mildly hypersusceptible to BMS-986001 (0.1- to 0.2- fold), even in the presence of K65R (0.3- to 1.3-fold).
A new 48-week phase II dose-finding study is comparing once-daily doses of 100, 200, and 400 mg plus efavirenz and 3TC to a control arm of efavirenz, tenofovir, and 3TC.[57]
BMS-663068 (Attachment Inhibitor)
BMS-663038 is a gp-120 attachment inhibitor being developed by Bristol-Myers Squibb.
Although no new studies have been presented since CROI 2011,[58],[59] new studies are listed as enrolling.[60],[61] These include a 24-week phase II dose-finding study with BMS-663038 dosed at 400 mg or 800 mg twice daily, or 600 mg or 120 mg once daily in combination with raltegravir and tenofovir and compared to a four-drug combination of boosted atazanavir plus raltegravir and tenofovir.
The initial dose-finding study used various once- and twice-daily doses with and without ritonavir.
Cenicriviroc
Cenicriviroc (previously TBR-652) is an oral CCR5 inhibitor being developed by Tobira that has a PK profile that allows once-daily dosing, but requires coadministration with food. Cenicriviroc is also active against CCR2, which plays a role in the inflammatory and metabolic pathways, the clinical implications of which are unclear, but may include a potential benefit in future studies.
In 2010, results from an initial phase II dose-finding study reported viral-load reductions of 1.4–1.8 log with 50–150 mg.[62]
This year at CROI, interim PK data were presented for the first 25 patients enrolled (24 men, 1 woman) in a more recent 48-week phase II randomized dose-finding study of 100 mg and 200 mg doses using a new 50 mg formulation, with efavirenz as a control and tenofovir/FTC as background NRTIs for all groups. Preliminary results reported dose-proportional pharmacokinetics with average plasma concentrations between 55.6 ng/mL and 722 ng/mL (estimated IC50 is 13.1 ng/mL) and mean (CV%) Cmin values of 41.0 (64.8%) and 89.2 (59.3%) ng/mL for the 100 mg and 200 mg doses, respectively (based on trough at day 28 for 18 patients). Two patients withdrew due to protocol noncompliance and two due to tolerability, all prior to day 14. This study is still ongoing, and virological efficacy and safety data are still to be reported. Based on these results, the study will enroll the remaining 125 patients.[63]
Rilpivirine Long-Acting (LA) Formulation
The development of a nanosuspension formulation of the NNRTI rilpivirine that could be given by intramuscular injection was reported several years ago. A single-dose PK study in HIV-negative people presented at CROI this year reported prolonged exposure in plasma, genital, and rectal compartments, following single doses of 300, 600, or 1,200 mg.[64]
While rilpivirine-LA was highlighted for its potential to reduce reliance on daily adherence in the context of PrEP, it might present important options for HIV treatment as well; this would require other ARVs with a similar formulation to construct a combination. The lack of negative drug interactions between rilpivirine and raltegravir (also presented at CROI),[65] and the development of a similar formulation for S/GSK 1265744, are clearly of interest.[53]
A safety issue for long-acting formulations, especially in the absence of an antidote to rapidly eliminate the active compound in the event of a severe adverse reaction, might be covered by a period of oral dosing to confirm individual tolerability, especially as both integrase and NNRTI classes have been associated with hypersensitivity reactions.
A recent survey of 400 HIV-positive patients attending two U.S. clinics reported 61%, 72%, and 84% interest in ART injections based on weekly, biweekly, and monthly formulations, respectively, with higher interest in people with concerns about adherence, although 35% were also concerned about needle use.[66]
Apricitabine
Apricitabine is an NRTI that has been included in the previous two Pipeline reports largely on the basis of interesting phase IIb results from 2008, and the commitment, from the small Australian biotech company Avexa, that itself acquired development rights from Shire, to expand treatment options for people with multidrug resistance.
The compound is a cytidine analogue, similar to 3TC, that is dosed twice daily, with phase II results showing activity against M184V resistance, independent of the presence other nucleoside analogue mutations (TAM pathways, L74V, etc.), and also showing viral-load reductions of –0.7 log for people with three or more thymidine analogue mutations (TAMs).[67]
Theoretically, using several similar compounds with modest viral activity that could overcome aspects of drug resistance might still have a therapeutic role for people who have run though other options. Unfortunately, the regulatory complications of developing multiple experimental options have never been resolved.
Planned phase II/III studies have been stopped or withdrawn, and Avexa is still looking for financial partners to take development forward.[68] Avexa also has integrase molecules in preclinical development.
Ibalizumab
Ibalizumab (previously TMB-355 and TNX-355) is a monoclonal antibody now owned by TaiMed that was listed as in phase I studies in the first TAG Pipeline Report in 2003. While a new phase I study is listed as open to enroll treatment-experienced patients (a phase II was completed in between), it is probably reasonable to say that optimistic predictions for a breakthrough in the next year are, conservatively, likely to be slim.[69]
CMX157 and CTP-518
Over the last year, there have been no further updates on CMX157, an NRTI similar to tenofovir that reported interesting phase I efficacy data two years ago, or on the atazanavir-like protease inhibitor CTP-518 that was acquired by GSK for preclinical development in 2009.
Outlook for Fixed-Dose Combinations
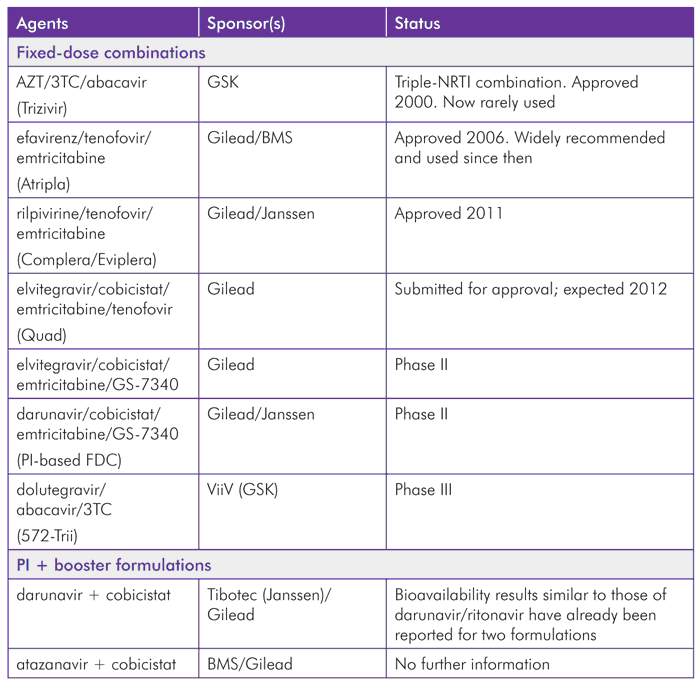
The optimistic outlook for fixed-dose combinations is summarized in Table 2.
With several combinations either already approved or in phase III development, the benefits to patient care from company collaboration are clearly an example that would help in other medical fields. The market is forcing Western companies to learn from generics: fixed-dose combinations simplified treatment for patients in developing countries with numerous formulations that have never been available when patents restricted this access.
The success of Atripla is well established, but also based on the efficacy of the three individual drugs it contains. Clearly, this will be just as important for future FDCs.
The potential for protease inhibitors to be coformulated with a PK booster is important, but requires companies to collaborate. Although ritonavir was originally approved in 1996, it was subsequently coformulated with Abbott’s own lopinavir in 2005, but Abbott did not license a stand-alone version until 2010, and has not coformulated it with protease inhibitors from other manufacturers that depend on boosting to reach therapeutic doses.
It is therefore helpful that agreements to co-formulate cobicistat with atazanavir and darunavir have been announced.[22],[23]
TABLE 2. Approved and Pipeline FDCs and Collaborations for Joint Formulations by Brand Manufacturer(s)
New Compounds of Interest
Although Gilead publicized its license of non-catalytic site integrase inhibitors (NCINIs) last year from Boehringer Ingelheim, including a lead compound BI 224436, the phase 1a dose-escalation study in HIV-negative volunteers has since been withdrawn.
Peptides that work similarly to integrase-like compounds (LEDGINs), while intriguing, are still in preclinical development.[70],[71]
Nanoformulations of existing ARVs still hold the same promise as they did in previous years’ reports—principally achieving higher targeted drug levels using compounds that require less API. For the most part, however, interesting compounds still remain in preclinical stages of development, though some proof-of-concept studies in humans are hoped for this year.
Cellular Transcription Factors
The potential for new targets is still a focus for earlier stages of drug development, and last year’s Pipeline briefly mentioned modification of antiviral human proteins including APOBEC3G, TRIM5-alpha, and tetherin that are active against HIV, but are neutralized by accessory HIV viral proteins.
Compounds that target HIV capsid include Tat inhibitors, RNase H inhibitors, gold-based compounds, tetherin (the protein that holds the virus to the host cell), and RN-18 (a compound that inhibits Vif and increases ABOBEC3G), and are still in preclinical studies.[72],[73]
Research on these compounds is closely connected to some of the strategies for cure research described by Richard Jefferys in another chapter of this publication, in the role that new drugs could play if designed to target reservoirs that are currently not reached by current ART. The existence of such sanctuary sites, while controversial, is clearly plausible, and supported by some research groups (though not by others).
In addition to designing drugs to target specific reservoirs, a study at CROI this year reported on the potential of APOBEC3G to intensify the impact of raltegravir.[74]
Conclusion
While in the last year only one new ARV compound was approved, several new compounds and FDCs are reaching regulatory stages, and this is expected to change treatment options, though this will also be dependent on how these drugs are priced in different markets.
It also remains to be seen whether the promising results reported in clinical trials, some of them involving less advanced patients, can be matched in routine or real-life settings, where patient characteristics are often very different.
These new drugs, while developed for a treatment-naive indication, also have promising activity for people with drug resistance, although some potentially useful compounds for resistant patients are still shelved waiting partnership with larger companies.
The potential development of treatments that work with human transcription factors and other cellular mechanisms still appears promising.
Finally, the industry outlook on new drugs, even at costs similar to or higher than those of current drugs, is sufficiently strong to ensure continued research in the near future. This is important. Current drugs are far from perfect, and an HIV cure, even if achievable, is optimistically suggested to be 10 to 20 years away.
Access to new treatments in anything other than the most financially wealthy settings (including some European countries) is less stable or certain, however, and this may result in further dividing treatment options in Western countries.
This might lead to a situation where pipeline compounds with very low milligram doses have the potential to become more widely used in developing rather than developed countries (depending on pricing policies for resource-limited settings) if the same manufacturers decide on premium pricing for new drugs for a Western market.
Postscript updates
Selected updates to the ARV pipeline will be posted below over the next year prior to the 2013 edition of the pipeline report.
June 2012
Elvitegravir: submitted to the FDA. Gilead PR, (2012 June 27). [75] Link to press release.
Cobicistat: submitted to the FDA. Gilead PR, (2012 June 28). [76] Link to press release.
July 2012
Dolutegravir: top-line results from phase 3 study reports in a company press release that dolutegravir was superior to Atripla in treatment naive patients, driven largely by reduced side effects. [77] Link to press release. Link to i-Base report.
Cobicistat: 48 week results reported cobicistat to be noninferior to ritonavir when boosting atazanavir in a phase 3 study in treatment naive patients. [78] Link to i-Base report. Link to abstract.
Dolutegravir: 48 week phase III results reported dolutegravir to be noninferior compared to raltegravir in treatment naive patients. [79] Link to i-Base report. Link to abstract.
Elvitegravir: 96 week phase III results reported continued noninferiority compared to raltegravir in treatment experienced patients. [80] Link to i-Base report. Link to abstract.
BMS 986001: Two posters at the IAS conference reported in vitro data supporting the safety profile of BMS 986001 to be distinct to d4T despite, supporting ongoing phase II studies. [81, 82] Link to i-Base report. Link to abstract 042. Link to abstract 041.
BMS 986001: A poster at the International Resistance workshop reported in vitro data on cross resistance profile of BMS 986001 that included hypersusceptibility to K65R and L74V (associated with resistance to tenofovir and abacavir respectively) but significantly reduced sensitivity to common thymidine analogue mutations. [83] Link to i-Base report.
August 2012
CMX157, EFdA and MK1439: Merck acquire two nucleosides and announces enrolment of a phase 2 dose-finding study for its in-house NNRTI MK1439. [84] Link to i-Base report.
Quad (elvitegravir/cobicistat/tenofovir/FTC): FDA announce approval for this 4-in-1 integrase inhibibitor-based single tablet fixed dose combination (FDC). [85] Gilead price Quad at 35% higher than Atripla, which will make impossible to access for most patients, especially for those receiving care in public health systems including the NHS. Link to i-Base report.
September 2012 – ICAAC updates
Dolutegravir: Week 48 results report superiority with abacavir/3TC compared to Atripla. The difference is driven by fewer discontinuations related to efavirenz side effects. [86] Link to i-Base report. Link to abstract.
S/GSK1265744: In vitro results on the resistance profile of this integrase inhibitor that is ViiV’s follow-up to dolutegravir. [87] Link to i-Base report. Link to abstract.
Albuvirtide: In vivo virological results of a long-acting HIV fusion inhibitor being developed in China. [88] Link to i-Base report. Link to abstract.
Elvitegravir/cobicistat has no clinically significant interactions with methadone or buprenorphine. Results from two small PK studies in patients on stable opiate substitution therapy (n=12 methadone; n=18 buprenorphine) reported no indications of an interaction between elvitegravir/cobicistat and methadone and only modestly increased buprenorphine levels that did not require dose modification. [89]
November 2012 – Glasgow
Quad: updated 96-week data were presented in two oral presentations at the Glasgow conference, with both supporting 48 week results. [89, 90] Link to i-Base report. Links to abstract: Oral abstract O424A and Oral abstract O424B.
December 2012
Dolutegravir: ViiV announces that dolutegravir is submitted to the E.U., U.S, and Canadian regulatory agencies. [91] Link to i-Base report. Link to press release.
February 2013
Lersivirine: ViiV announce that lersivirine development has been discontinued. This was not an unexpected decision. [92] This coincided with publication in JAIDS of 48 weeks results compared to efavirenz. [93] Link to i-Base report. Link to publication.
March 2013 – CROI 2013 updates
Dolutegravir: 24 week results from the phase 3 SAILING study in treatment experienced, integrase-naive patients reported 79% vs. 70% superiority compared to raltegravir, but this was an interim analysis and the primary endpoint is 48 weeks and background therapy was generally potent. [94] PK studies suggested that CSF penetration may be at therapeutic levels [95] and a lack of interactions between dolutegravir and methadone or oral contraceptives [96].
Tenofovir alafenamide (TAF, GS-7340): a phase 2 study comparing TAF vs TDF (each in a single pill formulation with elvitegravir/cobicistat/FTC) reported 87% vs 90% respectively for the primary endpoint of viral suppression <50 copies/mL at week 24. The results indicated a potential differences in favour of TAF relating to renal and bone toxicity markers, with no serious renal events in either arm. [97]
MK-1439: two studies at CROI presented data on this new NNRTI in development with Merck. In a 10-day monotherapy phase 1 study in 18 treatment naive men resulted in mean viral load reductions (90%CI) compared to placebo of –1.37 (–1.60, –1.14) and –1.26 (–1.51, –1.02) log copies/mL in the 25 and 200 mg arms, respectively, with non-significant differences between active doses at all time points. [98] Results from studies in HIV negative people reported lack of food interactions, supporting no early safety concerns. [99]
Cenicriviroc (TBR-652): a late breaker presentation of 24 week primary endpoint results from a phase 2b randomised placebo-controlled study of this CCR5/CCR2 inhibitor in 143 treatment naive patients reported approximately 76% and 73% vs 71% of patients in the 100 mg and 200 mg vs. efavrienz arms respectively. Tenovovir/FTC was used as background nucleosides by all participants. Daily pill count involved 6 tablets daily split into a morning and evening dose. There were fewer drug-related discontinuations in the cenicriviroc arms, but also more missing virologic data at week 24. Levels of sCD14 were lower in the cenicriviroc arms related to the inhibitory effect on CCR2. [100]
Maturation inhibitors: In vitro data was presented for second-generation maturation inhibitors that have the potential to overcome the most common natural polymorphism that reduced activity of bevirimat to less than 50% of HIV positive patients with drug resistance who are reliant on new drug classes. [101]
S/GSK1265744 LAP (long acting parenteral) formulation: monthly intramuscular injections protected eight macaques from serial rectal challenges. All control animals became infected within 2-7 weeks. Although this late breaker oral presentation was focussed on PrEP, it included early in vitro PK and efficacy data in humans, suggesting that monthly or even quarterly dosing may be possible for therapeutic use. [102]
Nanoformulations: a poster discussion [103] included several nanoformulations of currently approved ARVs including atazanavir/ritonavir [104, 105] and efavirenz [106]. The efavirenz formulation, in development at Liverpool University (they also have a lopinavir/ritonavir nanoformulation) is expected to enter first in-human studies later in 2013.
Sources
Information about clinical trials is based on the U.S.-based clinical trials registry (clinicaltrials.gov) and for study results on the online U.S. National Library of Medicine (pubmed.gov) current in May 2012, as a result of the following search terms:
APOBEC3G, apricitabine, BMS-986001, BMS-663068, cenicriviroc, cobicistat, CMX-157, CTP-518, dolutegravir, elvitegravir, GS-7340, GS-9137, GSK-1265744, ibalizumab, IDX-899, IDX-989, lersivirine, OBP-601, PF-3716539, rilpivirine, RN-18, SPI-251, TBR-652, tetherin, TMB-355, TMC-310991, TMC-558445, TNX-355, TRIM5-alpha.
Company press releases have been used for some updates, with the usual caveat that they may include forward-looking statements.
References
- Food and Drug Administration (U.S.) Approval of generic nevirapine formulations. 2012 May 22. Available from: http://www.fda.gov/ForConsumers/ByAudience/ForPatientAdvocates/HIVandAIDSActivities/ucm305654.htm. (Accessed 2012 June 26)
- Clotet B. Caring for HIV infected patients in Spain during the current economic crisis. Opening lecture presented at: 13th International Workshop on Clinical Pharmacology of HIV Therapy; 2012 April 16–18; Barcelona, Spain. Available from: http://regist2.virology-education.com/2012/13hivpk/docs/01_Clotet.pdf. (Accessed 2012 June 26)
- GBI Research. Antivirals market to 2017: increased uptake of high priced combination drugs will offset the impact of generics in the HIV therapeutics market. 2012 April. Available from: http://www.gbiresearch.com/Report.aspx?ID=Antivirals-Market-to-2017-Increased-Uptake-of-High-Priced-Combination-Drugs-will-Offset-the-Impact-of-Generics-in-the-HIV-Therapeutics-Market&ReportType=Industry_Report&Title=Pharmaceuticals_and_Healthcare. (Accessed 2012 June 26)
- Food and Drug Administration (U.S.). FDA approval of Complera: emtricitabine/rilpivirine/tenofovir DF fixed dose combination. 2011 August 10. Available from: http://www.fda.gov/ForConsumers/ByAudience/ForPatientAdvocates/HIVandAIDSActivities/ucm267592.htm. (Accessed 2012 June 26)
- Tibotec. Highlights of prescribing information for Edurant (rilpivirine) [tablets]. 2011 May. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/202022s000lbl.pdf. (Accessed 2012 June 26)
- DeJesus E, Rockstroh J, Henry K, et al. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate versus ritonavir-boosted atazanavir plus co-formulated emtricitabine and tenofovir disoproxil fumarate for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet. 2012 Jun 30;379(9835):2429–38. Abstract available from: http://www.thelancet.com/journals/lancet/article/PIIS0140-6736(12)60918-0. (Accessed 2012 July 2)
- Sax PE, DeJesus E, Mills A, et al. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus co-formulated efavirenz, emtricitabine, and tenofovir for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3 trial, analysis of results after 48 weeks. Lancet. 2012 Jun 30;379(9835):2439–48. Abstract available from: http://www.thelancet.com/journals/lancet/article/PIIS0140-6736(12)60917-9. (Accessed 2012 July 2)
- Gilead (Press Release). FDA advisory committee supports approval of Gilead’s once-daily Quad single tablet regimen for HIV. 2012 May 11. Available from: http://investors.gilead.com/phoenix.zhtml?c=69964&p=irol-newsArticle&ID=1695211. (Accessed 2012 June 26). Briefing document available from: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/AntiviralDrugsAdvisoryCommittee/UCM303397.pdf. (Accessed 2012 June 26)
- National Institutes of Health (U.S.). Study to evaluate switching from regimens consisting of a ritonavir-boosted protease inhibitor plus emtricitabine/tenofovir fixed-dose combination to the elvitegravir/cobicistat/emtricitabine/tenofovir DF single-tablet regimen in virologically suppressed, HIV 1 infected patients. Available from: http://clinicaltrials.gov/ct2/show/NCT01475838. (Accessed 2012 June 26)
- National Institutes of Health (U.S.). Phase 3b open label study to evaluate switching from regimens consisting of a NNRTI plus emtricitabine and tenofovir DF to the elvitegravir/cobicistat/emtricitabine/tenofovir DF single-tablet regimen in virologically suppressed, HIV 1 infected patients. Available from: http://clinicaltrials.gov/ct2/show/NCT01495702. (Accessed 2012 June 26)
- National Institutes of Health (U.S.). Open-label pilot study to evaluate switching from a regimen consisting of raltegravir plus emtricitabine/tenofovir DF fixed-dose combination to the elvitegravir/cobicistat/emtricitabine/tenofovir DF single-tablet regimen in virologically suppressed, HIV-1 infected patients. Available from: http://clinicaltrials.gov/ct2/show/NCT01533259. (Accessed 2012 June 26)
- DeJesus E, Berger D, Markowitz M, et al. Antiviral activity, pharmacokinetics, and dose response of the HIV-1 integrase inhibitor GS-9137 (JTK-303) in treatment-naive and treatment-experienced patients. J Acquir Immune Defic Syndr. 2006 Sep;43(1):1–5. Available from: http://journals.lww.com/jaids/Fulltext/2006/09000/Antiviral_Activity,_Pharmacokinetics,_and_Dose.1.aspx. (Accessed 2012 June 26)
- Ramanathan S, Mathias AA, German P, et al. Clinical pharmacokinetic and pharmacodynamic profile of the HIV integrase inhibitor elvitegravir. Clin Pharmacokinet. 2011 Apr;50(4):229–44.
- Ramanathan S, Wang H, Stondell T, et al. Pharmacokinetics and drug interaction profile of cobicistat boosted-EVG with atazanavir, rosuvastatin or rifabutin (Abstract O_03). Paper presented at: 13th International Workshop on Clinical Pharmacology of HIV Therapy; 2012 April 16–18; Barcelona, Spain. Published in Reviews in Antiviral Therapy & Infectious Diseases. 2012;3:5. Available from: http://regist2.virology-education.com/abstractbook/2012_3.pdf; and http://regist2.virology-education.com/2012/13hivpk/docs/05_Ramanathan.pdf. (Accessed 2012 June 26)
- National Institutes of Health (U.S.). Multicenter, randomized, double-blind, double-dummy, phase 3 study of the safety and efficacy of ritonavir-boosted elvitegravir (EVG/r) versus raltegravir (RAL). Available from: http://clinicaltrials.gov/ct2/show/NCT00708162. (Accessed 2012 June 26).
- Gilead (Press Release). Gilead submits new drug application to U.S. FDA for HIV integrase inhibitor elvitegravir for treatment-experienced patients. 2012 June 27. Available from: http://www.gilead.com/pr_1709769. (Accessed 2012 July 2)
- Mathias AA, German P, Murray BP, et al. Pharmacokinetics and pharmacodynamics of GS-9350: a novel pharmacokinetic enhancer without anti-HIV activity. Clin Pharmacol Ther. 2010 Mar;87(3):322–9.
- National Institutes of Health (U.S.). Safety and efficacy of GS-9350-boosted atazanavir compared to ritonavir-boosted atazanavir in combination with emtricitabine/tenofovir disoproxil fumarate in HIV-1 infected, antiretroviral treatment-naive adults. Available from: http://clinicaltrials.gov/ct2/show/NCT00892437. (Accessed 2012 June 26)
- German P, Liu HC, Szwarcberg J, et al. Effect of cobicistat on glomerular filtration rate in subjects with normal and impaired renal function. J Acquir Immune Defic Syndr. 2012 Jul 1;60(3):219–20. Abstract available from: http://journals.lww.com/jaids/Abstract/publishahead/Effect_of_Cobicistat_on_Glomerular_Filtration_Rate.98474.aspx. (Accessed 2012 July 2)
- National Institutes of Health (U.S.). Phase 3, randomized, double-blind study to evaluate the safety and efficacy of GS-9350-boosted atazanavir versus ritonavir-boosted atazanavir each administered with emtricitabine/tenofovir disoproxil fumarate in HIV-1 infected, antiretroviral treatment-naïve adults. Available from: http://clinicaltrials.gov/ct2/show/NCT01108510. (Accessed 2012 June 26)
- National Institutes of Health (U.S.). Phase 3b open-label, single arm study to evaluate the safety and efficacy of cobicistat-boosted darunavir in HIV infected adults. Available from: http://clinicaltrials.gov/ct2/show/NCT01440569. (Accessed 2012 June 26)
- Gilead (Press Release). Bristol-Myers Squibb and Gilead Sciences announce licensing agreement for development and commercialization of new fixed-dose combination pill for people living with HIV. 2011 October 26. Available from: http://www.gilead.com/pr_1621754. (Accessed 2012 June 26)
- Gilead (Press Release). Gilead Sciences announces agreement with Tibotec Pharmaceuticals to develop and commercialize a new fixed-dose combination of cobicistat and Prezista(r). 2011 June 28. Available from: http://www.gilead.com/pr_1580287. (Accessed 2012 June 26)
- Ramanathan S, Wang H, Szwarcberg J, et al. Safety/tolerability, pharmacokinetics, and boosting of twice-daily cobicistat administered alone or in combination with darunavir or tipranavir (Abstract P_08). Poster session presented at: 13th International Workshop on Clinical Pharmacology of HIV Therapy; 2012 April 16—18; Barcelona, Spain. Published in Reviews in Antiviral Therapy & Infectious Diseases. 2012;3:34–35. Available from: http://regist2.virology-education.com/abstractbook/2012_3.pdf. (Accessed 2012 June 26)
- Kakuda TN, Opsomer M, Timmers M, et al. Bioavailability of two FDC formulations of darunavir/cobicistat 800/150mg compared with darunavir/ritonavir 800/100mg co-administered as single agents (Abstract O_20). Paper presented at: 13th International Workshop on Clinical Pharmacology of HIV Therapy; 2012 April 16–18; Barcelona, Spain. Available from: http://www.hiv-druginteractions.org/data/NewsItem/94_13%20PKW%20Barcelona.pdf. (Accessed 2012 June 26)
- National Institutes of Health (U.S.). Cobicistat-containing highly active antiretroviral regimens in HIV-1 infected patients with mild to moderate renal impairment. Available from: http://clinicaltrials.gov/ct2/show/NCT01363011. (Accessed 2012 June 26)
- Gilead (Press Release). Gilead submits new drug application to U.S. FDA for boosting agent cobicistat. 2012 June 28. Available from: http://www.gilead.com/pr_1710422. (Accessed 2012 July 2)
- Ryom L; the D:A:D Study Group. Exposure to ARV and the risk of renal impairment among HIV+ persons with normal baseline renal function: the D:A:D study (Abstract 865). Poster session presented at: 19th Conference on Retroviruses and Opportunistic Infections; 2012 March 5–8; Seattle, WA. Available from: http://www.retroconference.org/2012b/Abstracts/45437.htm; and http://www.retroconference.org/2012b/PDFs/865.pdf. (Accessed 2012 June 26)
- Scherzer R, Estrella M, Li Y, et al. Association of tenofovir exposure with kidney disease risk in HIV infection. AIDS. 2012 Apr 24;26(7):867–75. Available from: http://journals.lww.com/aidsonline/Fulltext/2012/04240/Association_of_tenofovir_exposure_with_kidney.12.aspx. (Accessed 2012 June 26)
- Markowitz M, Zolopa A, Ruane P, et al. GS-7340 demonstrates greater declines in HIV-1 RNA than TDF during 14 days of monotherapy in HIV-1-infected subjects (Abstract 152LB). Paper presented at: 18th Conference on Retroviruses and Opportunistic Infections; 2011 February 27–March 3; Boston, MA. Available from: http://www.retroconference.org/2011/Abstracts/42549.htm. (Accessed 2012 June 26)
- Ruane P, DeJesus E, Berger D, et al. GS-7340 25 mg and 40 mg demonstrate superior efficacy to tenofovir 300 mg in a 10-day monotherapy study of HIV-1+ patients (Abstract 103). Paper presented at: 19th Conference on Retroviruses and Opportunistic Infections; 2012 March 5–8; Seattle, WA. Available from: http://www.retroconference.org/2012b/Abstracts/44081.htm. (Accessed 2012 June 26)
- Ramanathan S, Wei X, Custodio J, et al. Pharmacokinetics of a novel EVG/COBI/FTC/GS-7340 single tablet regimen (Abstract O_13). Paper presented at: 13th International Workshop on Clinical Pharmacology of HIV Therapy; 2012 April 16–18; Barcelona, Spain. Published in Reviews in Antiviral Therapy & Infectious Diseases. 2012;3:15. Available from: http://regist2.virology-education.com/abstractbook/2012_3.pdf. (Accessed 2012 June 26)
- National Institutes of Health (U.S.). Safety and efficacy of elvitegravir/cobicistat/emtricitabine/GS-7340 single tablet regimen versus elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate single tablet regimen in HIV 1 infected, antiretroviral treatment-naive adults. Available from: http://clinicaltrials.gov/ct2/show/NCT01497899. (Accessed 2012 June 26)
- National Institutes of Health (U.S.). Safety and efficacy of darunavir/cobicistat/emtricitabine/GS-7340 single tablet regimen versus cobicistat-boosted darunavir plus emtricitabine/tenofovir disoproxil fumarate fixed dose combination in HIV-1 infected, antiretroviral treatment naive adults. Available from: http://clinicaltrials.gov/ct2/show/NCT01565850. (Accessed 2012 June 26)
- Vandekerckhove L. GSK-1349572, a novel integrase inhibitor for the treatment of HIV infection. Curr Opin Investig Drugs. 2010 Feb;11(2):203–12.
- Dooley K, Purdy E, Sayre P, et al. Safety, tolerability, and pharmacokinetics of the HIV integrase inhibitor dolutegravir given twice daily with rifampin: results of a phase I study among healthy subjects (Abstract 148). Paper presented at: 19th Conference on Retroviruses and Opportunistic Infections; 2012 March 5–8; Seattle, WA. Available from: http://www.retroconference.org/2012b/Abstracts/43754.htm. (Accessed 2012 June 26)
- Song I, Borland J, Chen S, et al. Metabolism and drug-drug interaction profile of dolutegravir (DTG, S/GSK1349572) (Abstract O_07). Paper presented at: 13th International Workshop on Clinical Pharmacology of HIV Therapy; 2012 April 16–18; Barcelona, Spain. Available from: http://regist2.virology-education.com/abstractbook/2012_3.pdf. (Accessed 2012 June 26)
- Stellbrink HJ, Reynes J, Lazzarin A, et al. Dolutegravir in combination therapy exhibits rapid and sustained antiviral response in ARV-naïve adults: 96-week results from SPRING-1 (ING112276) (Abstract 102LB). Paper presented at: 19th Conference on Retroviruses and Opportunistic Infections; 2012 March 5–8; Seattle, WA. Available from: http://www.retroconference.org/2012b/Abstracts/45432.htm. (Accessed 2012 June 26)
- Shionogi – ViiV Healthcare LLC (Press Release). Shionogi – ViiV Healthcare announces initial data from pivotal phase III study of dolutegravir in HIV. 2012 April 2. Available from: http://www.viivhealthcare.com/media-room/press-releases/2012-04-02.aspx?sc_lang=en. (Accessed 2012 June 26)
- National Institutes of Health (U.S.). Relative bioavailability study of two new dolutegravir/abacavir/lamivudine fixed dose combination tablets. Available from: http://clinicaltrials.gov/ct2/show/NCT01366547. (Accessed 2012 June 26)
- Patel P, Song I, Borland J, et al. Pharmacokinetics of a dolutegravir pediatric granule formulation in healthy adult subjects (Abstract 985). Poster session presented at: 19th Conference on Retroviruses and Opportunistic Infections; 2012 March 5–8; Seattle, WA. Available from: http://www.retroconference.org/2012b/Abstracts/44121.htm. (Accessed 2012 June 26). (Accessed 2012 June 26)
- Shionogi – Viiv Healthcare LLC. Welcome to the dolutegravir expanded access program (DEAP) Treatment Center. Available from: http://www.dolutegravir-eap.com. (Accessed 2012 June 26)
- National Institutes of Health (U.S.). Dolutegravir expanded access study (DEAP). Available from: http://clinicaltrials.gov/ct2/show/NCT01536873. (Accessed 2012 June 26)
- AIDS Community Research Initiative of America. Activists caution HIV+ patients and their physicians about monotherapy in upcoming access program. 2012 February 9. Available from: http://www.acria.org/content/activists-advise-caution-about-access-program. (Accessed 2012 June 26)
- National Institutes of Health (U.S.). A study to assess dolutegravir in HIV-infected subjects with treatment failure on an integrase inhibitor containing regimen. (VIKING-3). Available from: http://clinicaltrials.gov/ct2/show/NCT01328041. (Accessed 2012 June 26)
- National Institutes of Health (U.S.). Study assessing dolutegravir in HIV-1 infected subjects with virus resistant to raltegravir and/or elivitegravir (VIKING-4). Available from: http://clinicaltrials.gov/ct2/show/NCT01568892. (Accessed 2012 June 26)
- National Institutes of Health (U.S.). A trial comparing GSK1349572 50 mg plus abacavir/lamivudine once daily to Atripla (also called the SINGLE trial). Available from: http://clinicaltrials.gov/ct2/show/NCT01263015. (Accessed 2012 June 26).
- National Institutes of Health (U.S.). Dolutegravir compared to darunavir/ritonavir each in combination with dual nucleoside reverse transcriptase inhibitors (NRTIs) in ART-naive subjects (FLAMINGO). Available from: http://clinicaltrials.gov/ct2/show/NCT01449929. (Accessed 2012 June 26)
- Eron J, Durant J, Poizot-Martin I, et al. Activity of a next generation integrase inhibitor (INI), S/GSK1349572, in subjects with HIV exhibiting raltegravir resistance: initial results of VIKING study (ING112961) (Abstract MOAB0105). Paper presented at: 18th International AIDS Conference; 2010 July 18–23; Vienna, Austria. Available from: http://pag.aids2010.org/Abstracts.aspx?SID=631&AID=12762. (Accessed 2012 June 26)
- Eron J, Kumar P, Lazzarin A, et al. DTG in subjects with HIV exhibiting RAL resistance: functional monotherapy results of VIKING study cohort II (Abstract 151LB). Paper presented at: 18th Conference on Retroviruses and Opportunistic Infections; 2012 February 27–March 3; Boston, MA. Available from: http://www.retroconference.org/2011/Abstracts/42541.htm. (Accessed 2012 June 26)
- GlaxoSmithKline (Press Release). Antiviral activity of S/GSK1265744, a once-daily, unboosted integrase inhibitor in clinical development, evaluated in Phase 1-2a study in healthy and HIV-infected subjects. 2009 September 14. Available from: http://www.gsk.com/media/pressreleases/2009/2009_pressrelease_10106.htm. (Accessed 2012 June 26)
- National Institutes of Health (U.S.). A single dose escalation study to investigate the safety, tolerability and pharmacokinetics of intramuscular and subcutaneous long acting GSK1265744 in healthy subjects. Available from: http://clinicaltrials.gov/ct2/show/NCT01215006. (Accessed 2012 June 26)
- National Institutes of Health (U.S.). A study to investigate the safety, tolerability and pharmacokinetics of repeat dose administration of long-acting GSK1265744 and long-acting TMC278 intramuscular and subcutaneous injections in healthy adult subjects. Available from: http://clinicaltrials.gov/ct2/show/NCT01593046. (Accessed 2012 June 26)
- Vernazza P, Wang C, Pozniak A, et al. Efficacy and safety of lersivirine (UK-453,061) vs. efavirenz in antiretroviral treatment-naïve HIV-1-infected patients: week 48 primary analysis results from an ongoing, multicentre, randomised, double-blind, phase IIb trial (study A5271015) (Abstract TUAB0101). Paper presented at: 6th IAS Conference on HIV Pathogenesis, Treatment and Prevention; 2011 July 17–20; Rome, Italy. Available from: http://pag.ias2011.org/Abstracts.aspx?SID=55&AID=3950. (Accessed 2012 June 26)
- National Institutes of Health (U.S.). A long term safety study of lersivirine for the treatment of HIV-1 infection in subjects who have completed treatment with lersivirine in studies A5271015 and A5271022. Available from: http://clinicaltrials.gov/ct2/show/NCT01254656. (Accessed 2012 June 26)
- Hwang C, Zhu L, Chan H, et al. Antiviral activity, exposure-response, and resistance analyses of monotherapy with the novel HIV NRTI BMS-986001 in ART-experienced subjects (Abstract O_06). Paper presented at: 13th International Workshop on Clinical Pharmacology of HIV Therapy; 2012 April 16–18; Barcelona, Spain. Published in Reviews in Antiviral Therapy & Infectious Diseases. 2012;3:8. Available from: http://regist2.virology-education.com/abstractbook/2012_3.pdf. (Accessed 2012 June 26)
- National Institutes of Health (U.S.). Safety, efficacy and dose-response study of BMS-986001 in subjects with HIV-1 infection who are treatment-naïve. Available from: http://clinicaltrials.gov/ct2/show/NCT01489046. (Accessed 2012 June 26)
- Nettles R, Schurmann D, Zhu L, et al. Pharmacodynamics, safety, and pharmacokinetics of BMS-663068: a potentially first-in-class oral HIV attachment inhibitor (Abstract 49). Paper presented at: 18th Conference on Retroviruses and Opportunistic Infections; 2012 February 27–March 3; Boston, MA. Available from: http://www.retroconference.org/2011/Abstracts/41942.htm. (Accessed 2012 June 26)
- Nowicka-Sans B, Gong Y-F, Ho H-T, et al. Antiviral activity of a new small molecule HIV-1 attachment inhibitor, BMS-626529, the parent of BMS663068 (Abstract 518). Poster session presented at: 18th Conference on Retroviruses and Opportunistic Infections; 2012 February 27–March 3; Boston, MA. Available from: http://www.retroconference.org/2011/Abstracts/41587.htm. (Accessed 2012 June 26)
- National Institutes of Health (U.S.). HIV attachment inhibitor to treat human immunodeficiency virus 1 (HIV-1) infections. Available from: http://clinicaltrials.gov/ct2/show/NCT01384734. (Accessed 2012 June 26)
- National Institutes of Health (U.S.). Safety, efficacy and dose-response study of BMS-986001 in subjects with HIV-1 infection who are treatment-naïve. Available from: http://clinicaltrials.gov/ct2/show/NCT01489046. (Accessed 2012 June 26)
- Marier JF, Trinh M, Pheng LH, et al. Pharmacokinetics and pharmacodynamics of TBR-652, a novel CCR5 antagonist, in HIV-1-infected, antiretroviral treatment-experienced, CCR5 antagonist-naive patients. Antimicrob Agents Chemother. 2011 Jun;55(6):2768–74. Available from: http://www.retroconference.org/2012b/PDFs/600.pdf. (Accessed 2012 June 26)
- National Institutes of Health (U.S.). Efficacy, safety, and tolerability of cenicriviroc (CVC) in combination with Truvada or Sustiva plus Truvada in HIV 1-infected, antiretroviral treatment-naïve, adult patients infected with only CCR5-tropic virus. Available from: http://clinicaltrials.gov/ct2/show/NCT01338883. (Accessed 2012 June 26)
- Jackson A, Else L, Tija J, et al. Rilpavirine-LA formulation: pharmacokinetics in plasma, genital tract in HIV negative females and rectum in males (Abstract 35). Paper presented at: 19th Conference on Retroviruses and Opportunistic Infections; 2012 March 5–8; Seattle, WA. Available from: http://www.retroconference.org/2012b/Abstracts/44600.htm. (Accessed 2012 June 26)
- Crauwels H, Stevens M, De La Rosa, G, et al. Absence of pharmacokinetic interaction between the NNRTI rilpivirine (TMC278) and the integrase inhibitor raltegravir (Abstract 617). Poster session presented at: 19th Conference on Retroviruses and Opportunistic Infections; 2012 March 5–8; Seattle, WA. Available from: http://www.retroconference.org/2012b/Abstracts/44415.htm. (Accessed 2012 June 26)
- Swindells S, Flexner CW, Williams JN, et al. Long-acting parenteral nanoformulated antiretroviral therapy: patient interest and attitudes (Abstract P_01). Poster session presented at: 13th International Workshop on Clinical Pharmacology of HIV Therapy; 2012 April 16–18; Barcelona, Spain. Published in Reviews in Antiviral Therapy & Infectious Diseases. 2012;3:29. Available from: http://regist2.virology-education.com/abstractbook/2012_3.pdf. (Accessed 2012 June 26)
- Cahn P, Altclas J, Martins M, et al. 48-week data from study AVX-201 – A randomised phase IIb study of apricitabine in treatment-experienced patients with M184V and NRTI resistance (Abstract O414). Paper presented at: Ninth International Congress on Drug Therapy in HIV Infection; 2008 November 9–13; Glasgow, United Kingdom. Available from: http://www.springerlink.com/content/j316274167445388/fulltext.pdf. (Accessed 2012 June 26)
- National Institutes of Health (U.S.). Clinical trials listing for apricitabine. Available from: http://clinicaltrials.gov/ct2/results?term=apricitabine. (Accessed 2012 June 26)
- National Institutes of Health (U.S.). Safety study of ibalizumab subcutaneous injection in healthy volunteers (TMB-108). Available from: http://clinicaltrials.gov/ct2/show/NCT01292174. (Accessed 2012 June 26)
- Desimmie B, Humbert M, Lescrinier E, et al. LEDGF/p75 qualifies as a cellular target for anti-HIV therapy (Abstract 526). Poster session presented at: 18th Conference on Retroviruses and Opportunistic Infections; 2012 February 27–March 3; Boston, MA. Available from: http://www.retroconference.org/2011/Abstracts/40173.htm. (Accessed 2012 June 26)
- Schrijvers R, De Rijck J, Demeulemeester J, et al. LEDGF/p75-independent HIV-1 replication demonstrates a role for HRP-2 and remains sensitive to inhibition by LEDGINs. PLoS Pathog. 2012 Mar;8(3):e1002558. Available from: http://www.plospathogens.org/article/info%3Adoi%2F10.1371%2Fjournal.ppat.1002558. (Accessed 2012 June 26)
- Ali A, Wang J, Nathans RS, et al. Synthesis and structure-activity relationship studies of HIV-1 virion infectivity factor (Vif) inhibitors that block viral replication. ChemMedChem. 2012 May 3. doi: 10.1002/cmdc.201200079.
- Malim MH, Bieniasz PD. HIV restriction factors and mechanisms of evasion. Cold Spring Harb Perspect Med. 2012 May;2(5):a006940.
- Song C, Donohue J, D’Aquila R, et al. APOBEC3G synergistically augments HIV-1 integrase inhibition by raltegravir (Abstract 252). Poster session presented at: 19th Conference on Retroviruses and Opportunistic Infections; 2012 March 5–8; Seattle, WA. Available from: http://retroconference.org/2012b/Abstracts/45477.htm. (Accessed 2012 June 26)
Postscript references
All weblinks were accessed at the date of the original posting. - Gilead PR. Gilead Submits New Drug Application to U.S. FDA for HIV Integrase Inhibitor Elvitegravir for Treatment-Experienced Patients. (27 June 2012). http://www.gilead.com/pr_1709769
- Gilead PR. Gilead ’s Boosting Agent Cobicistat for HIV Therapy as Effective as Ritonavir in Pivotal Phase 3 Study. (28 June 2012). http://www.gilead.com/pr_1717796
- Shionogi-ViiV Healthcare press release. Shionogi-ViiV Healthcare announces positive initial data from phase III study of dolutegravir-based regimen vs Atripla in HIV. (11 July 2012). http://www.viivhealthcare.com/media-room/press-releases/2012-07-11.aspx?sc_lang=en
- Gallant J et al. Cobicistat versus ritonavir as pharmacoenhancers in combination with atazanavir plus tenofovir disoproxil fumarate/emtricitabine: phase 3 randomized, double blind, active-controlled trial, week 48 results. 19th International AIDS Conference. 22-27 July 2012, Washington. Oral abstract TUAB0103.http://pag.aids2012.org/Abstracts.aspx?SID=202&AID=13085 ; http://pag.aids2012.org/flash.aspx?pid=1204
- Raffi F et al. Once-daily dolutegravir (DTG; S/GSK1349572) is non-inferior to raltegravir (RAL) in antiretroviral-naive adults: 48 week results from SPRING-2 (ING113086). 19th International AIDS Conference. 22-27 July 2012, Washington. Late breaker oral presentation THLBB04. http://pag.aids2012.org/abstracts.aspx?aid=20990 ; http://pag.aids2012.org/flash.aspx?pid=3888
- Elion R et al. Efficacy and safety results from a randomized, double blind, active controlled trial of elvitegravir (once-daily) versus raltegravir (twice-daily) in treatment-experienced HIV-positive patients: long term 96-week data. 19th International AIDS Conference. 22-27 July 2012, Washington. Oral abstract TUAB0105. http://pag.aids2012.org/Abstracts.aspx?SID=202&AID=5823 ; http://pag.aids2012.org/flash.aspx?pid=1386
- Wang F et al. The HIV NRTI BMS-986001 does not degrade mitochondrial DNA in long term primary cultures of cells isolated from human kidney, muscle and subcutaneous fat. 19th International AIDS Conference. 22-27 July 2012, Washington. Poster abstract TUPE042. http://pag.aids2012.org/abstracts.aspx?aid=17957
- Guha M et al. Absence of renal and bone toxicity in non-clinical studies of BMS-986001, a nucleoside reverse transcriptase inhibitor (NRTI) of human immunodeficiency virus (HIV). 19th International AIDS Conference. 22-27 July 2012, Washington. Poster abstract TUPE041. http://pag.aids2012.org/abstracts.aspx?aid=16832
- Li P et al. The in vitro cross-resistance profile of the NRTI BMS-986001 against known NRTI resistance mutations. 20th Intl Drug Resistance Workshop, 5–9 June 2012, Sitges. Abstract 2. Antiviral Therapy 2012: 17 Suppl 1:A10. http://www.intmedpress.com/journals/avt/abstract.cfm?id=2179&pid=88
- Merck press release. Merck signs two deals for novel HIV drug candidates and initiates phase II clinical trial of MK-1439 for HIV. (24 July 2012). http://www.merck.com/newsroom/news-release-archive/research-and-development/2012_0724.html ; http://i-base.info/htb/20318
- FDA press release: FDA approves new combination pill for HIV treatment for some patients. (27 August 2012). http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm317004.htm
- Walmsley S et al. Dolutegravir (DTG; S/GSK1349572) + abacavir/lamivudine once daily statistically superior to tenofovir/emtricitabine/efavirenz: 48-week results – SINGLE (ING114467). 52nd ICAAC, 9-12 September 2012, San Francisco. Abstract H-556b. http://www.abstractsonline.com/Plan/ViewAbstract.aspx?sKey=e1c18d5b-830f-4b4e-8671-35bcfb20eed5&cKey=af219b7d-2171-46b2-91ef-b8049552c9e5&mKey=%7b6B114A1D-85A4-4054-A83B-04D8B9B8749F%7d
- Yoshinaga T et al. Antiviral characteristics of S/GSK1265744, an HIV integrase inhibitor (INI) dosed by oral or long-acting parenteral Injection. 52nd ICAAC, San Francisco, 9-12 September 2012. Oral abstract H-550. http://www.abstractsonline.com/Plan/ViewAbstract.aspx?sKey=e1c18d5b-830f-4b4e-8671-35bcfb20eed5&cKey=3ecd6397-c9eb-4793-94f5-fde7784fe6ab&mKey=%7b6B114A1D-85A4-4054-A83B-04D8B9B8749F%7d
- Wu H et al. Albuvirtide, the first long-acting HIV fusion inhibitor, suppressed viral replication in HIV-infected adults. 52nd ICAAC, 9-12 September 2012, San Francisco. Abstract H554. http://www.abstractsonline.com/Plan/ViewAbstract.aspx?sKey=e1c18d5b-830f-4b4e-8671-35bcfb20eed5&cKey=70d14bcc-bad6-4754-b4b1-66b7d2559a23&mKey=%7b6B114A1D-85A4-4054-A83B-04D8B9B8749F%7d
- Zolopa A et al. Elvitegravir/cobicistat/emtricitabine/tenofovir DF (Quad) has durable efficacy and differentiated safety compared to efavirenz/emtricitabine/tenofovir DF at week 96 in treatment-naive HIV-1-infected patients. 11th International Congress on Drug Therapy in HIV, 11-15 November 2012, Glasgow. Oral abstract O424A.
- Fordyce M et al. Elvitegravir/cobicistat/emtricitabine/tenofovir DF (Quad) has durable efficacy and differentiated safety compared to atazanavir boosted by ritonavir plus emtricitabine/tenofovir DF at week 96 in treatment-naıve HIV-1-infected patients. 11th International Congress on Drug Therapy in HIV, 11-15 November 2012, Glasgow. Oral abstract O424B.
- ViiV press release. ViiV Healthcare announces regulatory submissions for dolutegravir in the EU, US and Canada. 17 December 2012. http://www.viivhealthcare.com/media-room/press-releases/2012-12-17
- ViiV stops development of NNRTI lersivirine. Link to i-Base report.
- Vernazza et al. Efficacy and safety of lersivirine (UK-453,061) versus efavirenz in antiretroviral treatment–naive HIV-1–infected patients: week 48 primary analysis results from an ongoing, multicenter, randomized, double-blind, phase IIb trial. JAIDS. 62(2):171-179. Published online ahead of print. February 1, 2013. doi: 10.1097/QAI.0b013e31827a2ba2. http://journals.lww.com/jaids/pages/results.aspx?k=lersivirine&Scope=AllIssues&txtKeywords=lersivirine
- Pozniak A et al. Dolutegravir vs raltegravir in ART-experienced, integrase-naïve subjects: 24-Week interim results from SAILING (ING111762). 20th CROI, 2013, Atlanta. Late breaker poster 179LB. Link to i-Base report. Link to abstract
- Letendre S et al. Distribution and antiviral activity in cerebrospinal fluid of the integrase inhibitor, dolutegravir: ING116070 week 16 results. 20th CROI, 2013, Atlanta. Late breaker poster 178LB. Link to i-Base report. Link to abstract.
- Song I et al. Dolutegravir has no effect on pharmacokinetics of methadone or Oral Contraceptives with Norgestimate and Ethinyl Estradiol. 20th CROI, 2013, Atlanta. Poster abstract 535. Link to i-Base report. Link to abstract.
- Zolopa A et al. Comparative study of tenofovir alafenamide vs tenofovir disoproxil fumarate, each with elvitegravir, cobicistat, and emtricitabine, for HIV treatment. 20th CROI, 2013, Atlanta. Late breaker oral abstract 99LB. Link to i-Base report. Link to abstract.
- Anderson M et al. Safety and antiviral activity of MK-1439, a novel NNRTI, in treatment-naïve HIV+ patients. 20th CROI, 2013, Atlanta. Oral abstract 100. Link to i-Base report. Link to abstract.
- Anderson M et al. Safety, tolerability, and pharmacokinetics of single and multiple doses of MK-1439, a novel HIV NNRTI, in healthy subjects. 20th CROI, 2013, Atlanta. Poster abstract 527. Link to i-Base report. Link to abstract.
- Gathe J et al. Week-24 primary analysis of cenicriviroc vs efavirenz, in combination with emtricitabine/tenofovir, in treatment-naïve HIV-1+ adults with CCR5-tropic virus. 20th CROI, 2013, Atlanta. Late breaker oral abstract 106LB. Link to i-Base report. Link to abstract.
- Urano E et al. Potent antiviral activity of 2nd generation maturation inhibitors against bevirimat-resistant polymorphic HIV-1 isolates. 20th CROI, 2013, Atlanta. Oral poster 105. Link to i-Base report. Link to abstract.
- Andrews C et al. Long-acting Parenteral Formulation of GSK1265744 Protects Macaques against Repeated Intrarectal Challenges with SHIV. Oral late breaker abstract 24LB. Link to i-Base report. Link to abstract.
- New Approaches to ARV Drug Delivery. Themed Poster Discussion. Monday 4 March 2013 1:30-2:30 pm. Link to i-Base report. Link to session.
- Puligujj P et al. Improved biodistribution, pharmacokinetics, and ARV responses for folate-targeted nanoformulated ART. 20th CROI, 2013, Atlanta. Poster abstract 513. Link to i-Base report. Link to abstract.
- Guo D et al. Development of Small Magnetite ARV Nanoparticles for Targeted Drug Delivery to Viral Reservoirs. 20th CROI, 2013, Atlanta. Poster abstract 512b. Link to i-Base report. Link to abstract.
- Liptrott N et al. Enhanced pharmacological properties of efavirenz formulated as solid drug nanoparticles. 20th CROI, 2013, Atlanta. Poster Poster abstract 512a. Link to i-Base report. Link to abstract.
