Generic drug registration, licensing, and a trip to Gilead’s islands
Karyn Kaplan and Tracy Swan
Access to essential medicines is part of the human right to health. The HIV/AIDS epidemic has demonstrated that generic competition is key to massive antiretroviral treatment (ART) scale-up in low- and middle-income countries (LMICs). But several steps are needed to create access to generics, including registration and licensing. Understanding these steps is critical for effective advocacy.
Patent protection, which extends for at least 20 years, keeps medicines for HIV, tuberculosis, and viral hepatitis unaffordable. Access to generic drugs is lifesaving: according to the World Health Organization, generic antiretrovirals have helped to avert more than 4 million HIV-related deaths in LMICs.
Médecins Sans Frontières (MSF) has documented the impact of generic competition on drug prices. Within a decade, there has been a 99 percent price reduction for first-line ART—from US$10,000 to US$70—and prices continue to fall.
Access barriers can be overcome by reforming intellectual property laws to prevent patent monopolies. Some countries do not grant patents on new drugs; others include safeguards that protect public health when reforming their national patent law. Even where patents are granted, steps can be taken to increase access to medications. Registration and licensing are two paths to overcoming patent barriers.
Before drugs can be marketed, they must be registered—approved for use by national regulatory authorities. Registration policies and processes differ by country, but data on quality, safety, efficacy, and other characteristics of pharmaceutical products usually must be provided. Some regulatory authorities accept data from trials conducted in other countries, but others require originator and generic drug producers to conduct local studies.
Generic drugs must demonstrate bioequivalence (see “A Drug by Any Other Name,” page 1), but a full clinical development program is not necessary (and delays registration of generics).
Licensing is another critical step in expanding access. Different types of licenses can be used to increase access to generic medications and drive down their prices.
VOLUNTARY LICENSE VS. COMPULSORY LICENSE
Voluntary licenses (VLs) are commercial rather than public health–based arrangements. Pharmaceutical patent holders grant VLs that allow another drug company to manufacture a generic version of their drug. In return, the patent holder sets conditions and may receive a fee or royalty.
VLs allow pharmaceutical patent holders to control the market by selecting the countries where VLs are granted—and where generic drugs can be sold. VLs can include additional restrictions, such as the number of people who can be treated, what drugs can be co-formulated, and which suppliers must be used for the active pharmaceutical ingredients (APIs) needed to make drugs.
Tiered pricing—when an originator company offers lower prices on its own drugs in certain countries—is another commercial strategy to control the market and maximize profit. Prices are not based on affordability for government health care programs, or for millions of people who must pay for their own health care, diagnostics, and medicine.
Voluntary licenses and tiered pricing have proved less effective than unrestricted generic competition in expanding universal access to affordable HIV medicines. With ART, VLs have included excessive royalty rates or limited where drugs can be sold.
International trade agreements include legal safeguards that allow countries to issue a compulsory license (CL) in certain circumstances, including protecting public health. Governments can issue a CL to allow production, exportation, or importation of a generic version of a patented drug—without consent from the patent holder—for noncommercial use in national public health care programs.
Compulsory licensing has come at great political cost to many of the LMICs that have implemented it. Countries may face political backlash, such as threats of trade sanctions or other punitive measures, usually by pharmaceutical companies or the U.S. government.
Case Study: Access to a Lifesaving Hepatitis C Drug
Most of the 185 million people with hepatitis C virus (HCV) live in middle-income countries (MICs). Each year, almost 500,000 people die from HCV-associated liver cancer or liver failure—although hepatitis C is curable. The standard of care for hepatitis C has improved dramatically: safe and effective oral drugs, called direct-acting antivirals (DAAs), have cured over 90 percent of people in clinical trials.
Sofosbuvir (Sovaldi) is a game-changing, once-daily nucleotide polymerase inhibitor from Gilead. Although it must be used with other drugs, sofosbuvir is effective for all HCV genotypes, and in people with cirrhosis or those coinfected with HIV and HCV. In the United States, Gilead charges US$84,000 for a three-month course of sofosbuvir—about US$1,000 a pill. Activists, patients, clinicians, insurers, and U.S. senators alike deplore the price of the drug, which has limited availability in the U.S., the European Union, and Australia.
The pharmaceutical industry sees MICs as emerging markets, although they have the greatest income inequality. Sofosbuvir is needed most in MICs, where HCV and poverty are rampant. MICs are home to 73 percent of the world’s poorest people, and to 130 million people with hepatitis C.
In MICs, most people must pay for their own health care. Sofosbuvir (and other DAAs with which it must be used) must be affordable—or millions of people will continue to die from HCV.
 Activists have begun to fight for affordable DAAs. In February, during the first-ever Hepatitis C World Community Advisory Board meeting in Bangkok, activists—many from LMICs—met with representatives from AbbVie, Bristol-Myers Squibb, Gilead, Janssen, Merck, and Roche to press for access and discuss registration and licensing in their countries. A report from the meeting, Pills Cost Pennies, Greed Costs Lives, is available here.
Activists have begun to fight for affordable DAAs. In February, during the first-ever Hepatitis C World Community Advisory Board meeting in Bangkok, activists—many from LMICs—met with representatives from AbbVie, Bristol-Myers Squibb, Gilead, Janssen, Merck, and Roche to press for access and discuss registration and licensing in their countries. A report from the meeting, Pills Cost Pennies, Greed Costs Lives, is available here.
So far, Gilead has registered and licensed sofosbuvir in only one middle-income country, Egypt, where Gilead is selling it to the government for US$300 per month. Prices will be much higher—and unaffordable—for uninsured Egyptians, who must pay for their own medication; according to the World Bank, Egypt’s per capita annual GDP is US$3,314, but the expected private-market price will be US$9,000 for a 12-week course.
Gilead’s “Egypt price” sounds like a bargain—but it isn’t. Sofosbuvir can be mass-produced, at a profit, for far less than Gilead is charging anywhere. According to Andrew Hill, PhD, of the University of Liverpool and colleagues, three months of sofosbuvir could be mass-produced at a profit, and sold for as little as US$105. In September 2014, Gilead announced licensing agreements for generic sofosbuvir in 91 LMICs. The countries that are not included in these licenses must buy higher-priced sofosbuvir from Gilead. Limiting the countries where generic sofosbuvir can be sold will make it difficult for producers to reduce the price, because they cannot achieve economies of scale.
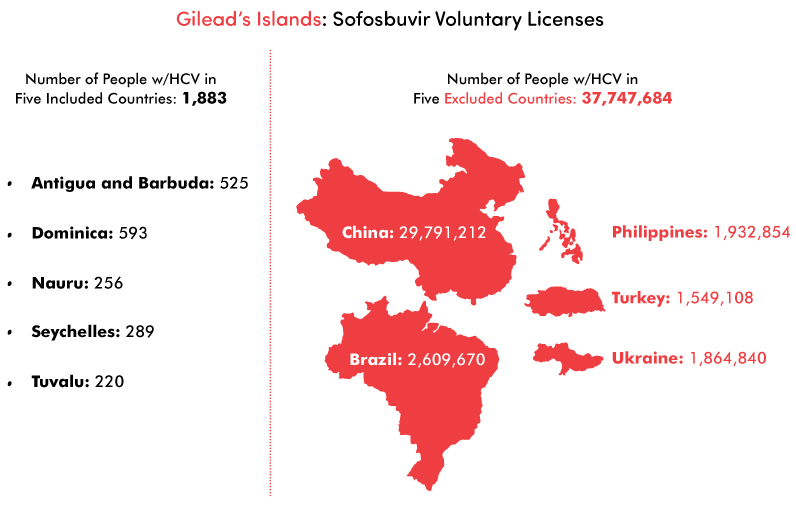
Gilead’s website features a section on developing-world access, which states: “We focus on the geographic and therapeutic areas where the company and its medicines can make the greatest difference.” It is difficult to understand how this principle informed Gilead’s selection process for sofosbuvir licenses, since it leaves out many countries with the highest numbers of people with HCV. Gilead did not offer licenses for generic sofosbuvir to five of the 20 countries with the largest number of hepatitis C cases (China, Brazil, the Philippines, Ukraine, and Turkey): approximately 38 million people. Instead, Gilead chose sparsely populated countries with smaller epidemics, such as Antigua and Barbuda, Dominica, Nauru, Seychelles, and Tuvalu—where less than 2,000 people have hepatitis C. This is a common industry tactic: beefing up the scope of the license for public relations purposes, rather than targeting countries with the most need.
Drug company–driven initiatives such as tiered pricing and voluntary licensing have demonstrated insufficient benefit, and act as a barrier to universal access to essential medications compared with unfettered generic drug competition.
Recommendations:
- Originator companies should register their drugs in all countries where there are people living with the disease.
- Generics producers should reject restrictive licenses.
- Governments should use the full range of legal options—such as issuing CLs—that are guaranteed in international trade agreements.
- The World Health Organization must actively promote and support governments’ use of these flexibilities and help countries to incorporate them into national law.
For information about ongoing HCV treatment access advocacy and to get involved in campaigns, please visit www.hepcoalition.org.
