Download:

By Erica Lessem
September 2014
I. Introduction
Tuberculosis (TB) continues to kill over a million people each year, and multidrug-resistant (MDR) TB is a growing problem with few treatment options. In April 2014, delamanid was approved by the European Medicines Agency (EMA) for the treatment of TB, becoming only the second new drug from a new drug class (after bedaquiline) to receive such approval in over forty years.1 Delamanid was also approved by the Japanese regulatory authority in July 2014.2 Delamanid (also known by its trade name, Deltyba, or formerly as OPC-67683) is an important option for people with MDR-TB who lack effective, tolerable treatments. This guide contains important safety and efficacy data on delamanid and offers advocacy recommendations for activists, including community representatives, advocates, clinicians, researchers, policy makers, donors, and private-sector employees.
II. Mechanism of Delamanid
Delamanid is a drug in the nitroimidazole class. Nitroimidazoles appear to kill TB bacteria by blocking the synthesis of mycolic acids (i.e., stopping the bacteria from creating building blocks important for their cell walls).3 Nitroimidazoles also appear to kill the TB bacteria by poisoning them with nitric oxide, which the drugs release when metabolized.4
Key Definitions and Acronyms
TB: tuberculosis
MDR-TB: multidrugresistant TB; or TB resistant to at least isoniazid and rifampin, the two most powerful existing TB drugs, which are used as part of the four-drug first-line therapy
Pre-XDR-TB: pre‑extensively drug-resistant TB; or MDR-TB that is resistant to either a secondline injectable drug (amikacin, kanamycin, or capreomycin) or a fluoroquinolone
XDR-TB: extensively drug-resistant TB; or MDRTB that is also resistant to a fluoroquinolone and at least one injectable second-line drug
III. Efficacy of Delamanid
EMA approval was based primarily on a two-month, phase II study involving 481 people with drugresistant TB (including both MDR-TB and XDR-TB). Participants were randomized to three arms: delamanid at 100 mg twice daily plus other available drugs in an optimized background regimen (OBR); delamanid at 200 mg twice daily plus an OBR; or placebo plus an OBR. This study found that:
- Delamanid, given for two months with an OBR, increased by 16 percent the proportion of people who no longer had cultures of sputum that grew M. tuberculosis, the bacterium that causes TB disease (also known as sputum culture conversion) (45.4% vs. 29.6%, P = .008). This is an important sign that treatment is working (see figure 1);5
- Delamanid, when given with an OBR, reduced the time required to achieve sputum culture conversion (cultures became negative faster); and6
- Delamanid did not appear more effective when given at 200 mg rather than 100 mg twice daily.7
After this study finished, surviving participants could choose to enter a six-month extension study, where their doctors could choose to give them delamanid at either 100 mg or 200 mg.8 Time gaps between inclusion in the two-month and the six-month studies varied widely, with over one-third of participants waiting four months or more. Thus, some got delamanid for six months (if they had not gotten it in the two-month study), some got it for eight months with a break in the middle, and those who did not participate in the six-month study got delamanid for either two months or not at all.
The EMA decided to look at the follow-up data from as many people as possible who participated in the first or both studies. Two years after starting treatment, 75 percent of those who received delamanid for six months or more had no bacteria in their sputum (participants who chose the six-month study),9 compared with 55 percent who received delamanid for two months or less (participants who did not choose the six-month study). Only one percent of those who received long-term delamanid died, versus 8.3% of those who got two months of no delamanid (P < .001).10 However, the confusing study design, with many losses to follow-up, drop-outs, and deaths, makes it hard to know if these differences were due to delamanid or other factors, and the best dose and length of treatment remain unclear.
Delamanid’s phase III trial completed enrollment in late 2013, but given the long treatment and follow-up time required for TB trials, results may take years to become available. This trial, which gives delamanid at 100 mg twice daily for two months, and then at 200 mg once daily for four months (which should simplify dosing), will provide more data on the long-term efficacy of the drug. In the meantime, the randomized, two-month trial data show clearly that delamanid is effective at killing TB bacteria in people with MDR-TB, and the available longer-term data, though flawed, point to possible improvements of long-term treatment outcomes and survival.
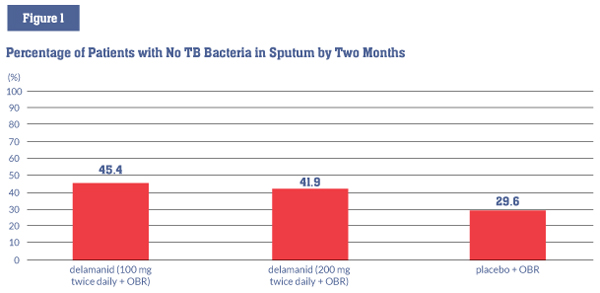
Note: The differences between the delamanid 100 mg and 200 mg groups and the placebo group were statistically significant (P = .008, and P = .04).
IV. Safety of Delamanid
Side effects and mortality
Delamanid appears to be a mostly safe drug with manageable side effects. Available data are insufficient to prove beyond a doubt an effect on mortality, due to confounding factors in the six-month study.
Delamanid’s most common side effects are:
- nausea, vomiting, and dizziness (in about one- third of people taking it);
- low potassium levels in the blood; and
- paresthesia (a pricking or tingling sensation), anxiety, and tremor (shaking).11
Delamanid’s most serious side effect is QT prolongation, a disturbance in the heart’s electrical activity that can lead to serious heart rhythm disturbances, such as ventricular tachycardia, and sometimes to sudden death. However, in the studies to date, no clinical events occurred as a result of this prolongation. Other than QT prolongation, the risk of having a serious side effect was about the same for patients receiving delamanid or placebo in the two-month trial.12 Delamanid did appear to have fewer side effects when given at 100 mg rather than 200 mg twice daily.13
Is delamanid safe and effective to use with other TB drugs?
Delamanid appears not to interact with most other TB drugs, though giving delamanid and ethambutol together increased the amount of ethambutol in the body by about 25 percent.14
QT prolongation, the most troubling side effect of delamanid, is also caused by other MDR-TB drugs like bedaquiline, clofazimine, and moxifloxacin. No studies have been done yet to show whether the effects of these drugs on heart rhythm are additive, or if the drugs are safe to use together (a U.S. National Institutes of Health AIDS Clinical Trials Group study will look at bedaquiline and delamanid together, but results will not be available till 2016). For patients with extensive drug resistance and limited treatment options, potential benefits of combining two or more of these drugs may outweigh potential risks, particularly when regular monitoring for heart safety (i.e., electrocardiograms, or ECGs) is available.
Is delamanid safe and effective to use with HIV medicines?
Delamanid has not been studied in people with HIV taking antiretrovirals. In a study of healthy participants, delamanid did not significantly affect the levels of tenofovir, lopinavir/ritonavir, or efavirenz in the body, though lopinavir/ritonavir did increase the amount of delamanid in the body by 20 percent.15,16 When the body breaks down delamanid, one particular molecule, DM-6705, appears to cause QT prolongation. Lopinavir/ritonavir seems to increase the amount of DM-6705 in the body by about 30 percent, and the EMA recommends frequent ECG monitoring if delamanid and lopinavir/ritonavir are taken together.17
Is delamanid safe for children, pregnant women, or nursing women?
Delamanid is currently in clinical trials for children ages six to 17 (children from birth to age six will be enrolled once safety data on older children and the pediatric formulation’s bioequivalence are available).18,19 While the trial is ongoing, the drug is recommended for adults only. In some circumstances, potential benefits may outweigh potential risks: delamanid has been given successfully to one 12-year-old child.20 Delamanid is not recommended for pregnant women, as animal studies have shown some toxicity to the fetus (when delamanid was given to pregnant rabbits at toxically high doses).21 However, it is also harmful to the fetus to have a mother who is very sick with TB. There may be circumstances (e.g., when other treatments are not effective, available, or tolerable) in which a pregnant woman decides that the potential benefit of taking delamanid outweighs the potential risk. Pregnant women have an absolute right to treatment and must be allowed to make informed decisions about their care in consultation with their doctors. It is unknown if delamanid is passed through human breast milk, so nursing mothers taking delamanid should consider discontinuing nursing.22
V. Access to Delamanid
EMA approval
Given the urgent need for new medicines to treat MDR-TB, the EMA granted approval based on the limited available data for delamanid for pulmonary MDR-TB (MDR-TB of the lungs) in adults for whom an effective treatment regimen cannot otherwise be constructed due to resistance or tolerability. The recommended dose is 100 mg twice daily, given for six months, in addition to other MDR-TB medicines. This approval of delamanid is conditional, meaning that Otsuka (the company that makes delamanid) must complete additional studies to maintain delamanid’s approvals (see table 1). Although the approval is for pulmonary MDR-TB, there is no reason to believe that the drug would not be effective in extrapulmonary MDR-TB (MDR-TB outside of the lungs).
Table 1. Activities Required by the EMA as a Condition of Delamanid’s Approval
| Requirement | Purpose | Deadline |
|---|---|---|
| Pediatric investigational plan* | Study the pharmacokinetics and safety of delamanid in children ages 0–18 (given for 10 days at 100 mg twice daily for children 12 and older, and 50 mg twice daily for children under 12, and then a six-month extension of that study to evaluate long-term safety and efficacy) and demonstrate bioequivalence of a pediatric dispersible formulation to the adult tablets | April 2017 |
| Phase III trial | Confirm delamanid’s safety and efficacy when given for six months (100 mg twice daily for two months, followed by 200 mg once daily for four months) at the beginning of 18–24 months of treatment with other MDR-TB drugs | First half of 2017 |
| Dosing study | Find the best dose of delamanid by comparing delamanid at 100 mg twice daily for two months, followed by 200 mg once daily for four months, with delamanid at 400 mg once daily | End of 2018 |
*Note: The EMA determined requirements for delamanid’s pediatric investigational plan in advance of delamanid’s approval.23
In theory, EMA approval gives Otsuka permission to market delamanid in all European Union countries. However, Otsuka has so far launched Deltyba only in the United Kingdom and Germany, where the drug is extremely expensive. In the United Kingdom, delamanid costs £1,045.83 for a 40-pack of 50 mg tablets (or about US$42.4124 per 50 mg tablet, or US$28,499 for a six-month course of 100 mg twice daily).25 In Germany, the 40-pack of 50 mg tablets costs €1,500 (or about US$49.47 per 50 mg tablet, or US$33,244 for a six-month course of 100 mg twice daily).26 Country-specific labeling and educational materials are not yet available for most countries, which may further delay access in other European countries that urgently need access to MDR-TB treatment options, such as Estonia, Latvia, Lithuania, and Romania.
Global access
Access to delamanid outside the European Union is even more challenging. Otsuka has only taken steps to register the drug in Japan, and has not filed or revealed a plan for filing for regulatory approval in any countries with high TB burdens or in the majority of countries that have participated in the clinical trials for delamanid’s EMA approval (China, Egypt, Korea, Moldova, Peru, the Philippines, and South Africa).
Furthermore, compassionate use access—a mechanism designed to give patients with urgent medical need access to a drug pre-approval—to delamanid has been severely delayed and unnecessarily limited. Compassionate use programs are vital for patients with XDR-TB, pre-XDR-TB, or serious challenges tolerating MDR-TB treatment. Otsuka initiated its compassionate use program only after EMA approval, despite years of requests from activists and clinicians. Even now that a compassionate use program has begun, many patients in need cannot get delamanid due to a lack of information about the drug’s existence, or due to restrictive policies. Otsuka refuses to grant access to delamanid to patients’ also being prescribed the new drug bedaquiline. This creates a life-threatening paradox for those in most dire need of drugs they can tolerate and to which their TB is susceptible: the more widespread the resistance and urgent the need for delamanid, the less likely Otsuka will be to grant it. Furthermore, Otsuka has refused to release information on how many requests have been initiated and granted under its compassionate use program.
Patients and providers interested in learning more about how to get delamanid in their country can e-mail erica.lessem@treatmentactiongroup.org. Providers interested in access to delamanid under compassionate use should send a request to medical@otsuka.de.
It is also unclear whether Otsuka has sufficient drug supply to meet the global demand. Limited awareness of and access to delamanid (given the company’s poor compassionate use and regulatory track record) mean that few requests for the drug have been made. But the global demand for safer, more effective MDR-TB treatment is large. Guidance from the World Health Organization on the use of delamanid is expected before the end of 2014, which will help inform countries and providers about the drug and may lead to increased demand.
VI. Take Action: Advocacy Messages
1. Delamanid is safe and is likely effective at treating MDR-TB.
While clinical trials of delamanid to date have had many limitations, more data are available to support the safety and efficacy of this drug against TB than there are for most MDR-TB drugs. Delamanid was not associated with an increase in mortality compared with placebo such as was seen with bedaquiline in phase II trials. The phase III trial to confirm delamanid’s safety and efficacy in a longterm, better-designed trial is completely enrolled. Patients with MDR-TB that is difficult to treat, such as pre-XDR-TB and XDR-TB, or intolerance to older drugs, need better options as soon as possible. As such:
- activists may want to advocate for the filing, approval, and use of delamanid in their countries now; or
- activists should plan for discussions with regulators and ministries of health once WHO guidance or phase III trial data are available.
2. High-quality research is urgently needed.
This is especially important given the poor design of delamanid’s six-month phase II trial and the limited populations in which Otsuka has studied the drug so far. Understanding the best way to use delamanid as part of a regimen is also crucial to improving care, as right now we have information on delamanid only when given in addition to a lengthy course of multidrug treatment. Analyses of acquired resistance to delamanid have been very limited, and further research is needed. To this end:
- Otsuka must complete a phase III trial and a dosing study of delamanid to confirm the drug’s safety, efficacy, and optimal dosing over six months;
- Otsuka should continue advancing its studies to determine delamanid’s safety and efficacy in infants and children;
- Otsuka should also conduct further research to determine delamanid’s safety and efficacy in people with HIV (especially those on antiretroviral therapy), people who use alcohol and drugs, people with extrapulmonary TB, people with XDR-TB, and pregnant women;
- Otsuka must collaborate with other companies, and with public and nonprofit TB drug trial sponsors, to establish the optimal use of delamanid. Studies must determine whether delamanid can safely and effectively improve care when used for longer periods of time; can shorten treatment overall; can be used with other new drugs like bedaquiline; or can replace other drugs that are more difficult to tolerate. Planning of these studies should advance even while results from the bedaquiline-delamanid drug-drug interaction study are pending;
- Otsuka must rigorously investigate the acquisition of resistance to delamanid; and
- Otsuka should also increase transparency of its trial designs in the planning process, engaging community members and scientific peers in protocol reviews, to ensure that research is high-quality, rigorous, and can definitively answer important questions (unlike delamanid’s six-month extension study), and reflects the needs of those affected by TB.
3. Widespread registration, better compassionate use policies, appropriate pricing, simple formulations, and robust drug supply are necessary to ensure timely approval and access to delamanid.
To ensure that those who need delamanid can get it:
- Otsuka must prioritize registering delamanid in countries with high MDR-TB burdens and countries that hosted the clinical trials contributing to delamanid’s EMA approval. Failure to do so is ethically unacceptable, as denying access to the populations that bore the burden of the research to prove the drug’s efficacy is a violation of the Declaration of Helsinki and will limit delamanid’s impact on TB;
- Otsuka must make its compassionate use program more widely accessible and formulate policies on when the potential benefits of giving delamanid and bedaquiline may outweigh potential risks, rather than citing an inflexible, illogical, and unjust position that the two can never be given together;
- Otsuka must price delamanid affordably and avoid the dangerous pitfall that tiered-pricing creates of overcharging middle-income countries, which shoulder most of the TB burden, thereby forcing programs with limited budgets to forego potentially lifesaving treatments or use suboptimal ones;
- Otsuka must also ensure that manufacturing capacity for delamanid is sufficient to meet global demand from the thousands of people who may benefit from this drug;
- Otsuka should also consider formulating delamanid in 100 mg pills to avoid the large pill burden that the current 50 mg formulation creates during twice-daily dosing of 100 mg (or four delamanid pills, on top of numerous other medicines); and
- overall, Otsuka must make its policies and plans more transparent.
Endnotes
- European Medicines Agency. Deltyba (delamanid): authorisation details. 2014 April 28. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002552/human_med_001699.jsp&mid=WC0b01ac058001d124. (Accessed 2014 August 26)
- Otsuka Pharmaceutical Co., Ltd. (Press Release). After 40 years, a new drug for the treatment of tuberculosis in Japan. 2014 July 4. Available from: http://www.otsuka.co.jp/en/company/release/2014/0704_02.html. (Accessed 2014 August 26)
- Matsumoto M, Hashizume H, Tomishige T, et al. OPC-67683, a nitro-dihydro-imidazooxazole derivative with promising action against tuberculosis in vitro and in mice. PLoS Med. 2006 Nov;3(11):e466. doi: 10.1371/journal.pmed.0030466.
- Singh R, Manjunatha U, Boshoff H, et al. PA-824 kills nonreplicating Mycobacterium tuberculosis by intracellular NO release. Science. 2008 Nov 28;322:1392–5. doi: 10.1126/science.1164571.
- Gler MT, Skripconoka V, Sanchez-Garavito E, et al. Delamanid for multidrug-resistant pulmonary tuberculosis. N Engl J Med. 2012 Jun 7;366(23):2151–60. doi: 10.1056/NEJMoa1112433.
- Ibid.
- Ibid.
- Skripconoka V, Danilovits M, Pehme L, et al. Delamanid improves outcomes and reduces mortality in multidrug-resistant tuberculosis. Eur Respir J. 2013 Jun;41(6):1393–400. doi: 10.1183/09031936.00125812.
- European Medicines Agency. EPAR summary for the public: Deltyba (delamanid). 2014 April 28. Available from: http://www.ema.europa.eu/docs/ en_GB/document_library/EPAR_-_Summary_for_the_public/human/002552/WC500166235.pdf. (Accessed 2014 August 8)
- Skripconoka V, et al. Delamanid improves outcomes.
- European Medicines Agency. EPAR summary for public.
- Gler MT, Skripconoka V, Sanchez-Garavito E, et al. Delamanid for multidrug-resistant pulmonary tuberculosis. N Engl J Med. 2012 Jun 7;366(23):2151–60. Supplementary appendix. Available from: http://www.nejm.org/doi/suppl/10.1056/NEJMoa1112433/suppl_file/nejmoa1112433_appendix.pdf. (Accessed 2014 September 15)
- Gler MT, et al. Delamanid for multidrug-resistant pulmonary tuberculosis.
- Otsuka Novel Products GmbH. Labelling and package leaflet: Deltyba. 2014 April. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002552/WC500166232.pdf. (Accessed 2014 August 8)
- Paccaly A, Petersen C, Patil S, et al. Absence of clinically relevant drug interaction between delamanid, a new drug for multidrug-resistant tuberculosis (MDR-TB) and tenofovir or lopinavir/ritonavir in health subjects. Poster session presented at: 19th International AIDS Conference; 2012 July 22–27; Washington, D.C.
- Peterson C., Paccaly A., Kim J., et al. Delamanid, a new drug for multi-drug resistant tuberculosis (MDR-TB), and efavirenz do not show clinically relevant drug interactions in healthy subjects. Paper presented at: 52nd Interscience Conference on Antimicrobial Agents and Chemotherapy; 2012 September 9–12; San Francisco, CA.
- Otsuka Novel Products GmbH. Labelling and package leaflet: Deltyba.
- ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (U.S.) 2000. Identifier NCT01859923, A 6-month safety, efficacy, and pharmacokinetic trial of delamanid in pediatric patients with multidrug resistant tuberculosis; 2013 May 15 (cited 2014 August 26). Available from: http://clinicaltrials.gov/ct2/show/NCT01859923?term=delamanid&rank=1.
- European Medicines Agency. European Medicines Agency decision P/0241/2012. 2012 October 22. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/PIP_decision/WC500135111.pdf. (Accessed 2014 August 26)
- Esposito S, D’Ambrosio L, Tadolini M, et al. ERS/WHO Tuberculosis Consilium assistance with extensively drug-resistant tuberculosis management in a child: case study of compassionate delamanid use. 2014 Sep;44(3):811–5. doi: 10.1183/09031936.00060414. Epub 2014 May 15.
- Otsuka Novel Products GmbH. Labelling and package leaflet: Deltyba. 22. Ibid.
- European Medicines Agency. European Medicines Agency decision P/0241/2012.
- We converted data reported in non–U.S. currency into U.S. dollars using the September 12, 2014, currency exchange rate provided by the OANDA Corporation: http://www.oanda.com/currency/converter.
- British National Formulary. Deltyba. Available from: http://www.evidence.nhs.uk/formulary/bnf/current/5-infections/51-antibacterialdrugs/519-antituberculosis-drugs/delamanid/deltyba (U.K. only) (Accessed 2014 August 26) and http://www.openproxy.co.uk/browse.php?u=Oi8vd3d3LmV2aWRlbmNlLm5ocy51ay9mb3JtdWxhcnkvYm5mL2N1cnJlbnQvNS1pbmZlY3Rpb25zLzUxLWFudGliYWN0ZXJpYWwtZHJ1Z3MvNTE5LWFudGl0dWJlcmN1bG9zaXMtZHJ1Z3MvZGVsYW1hbmlkL2RlbHR5YmE%3D&b=13&f=norefer. (Accessed 2014 September 15)
- MediPreis. Deltyba 50 mg filmtabletten. September 23, 2014 [cited 2014 August 26]. Available from: http://www.medipreis.de/preisvergleich/Deltyba-50-Mg-Filmtabletten-40-Stueck-Otsuka-Novel-Products-GmbH-10286871?preiseversand.
