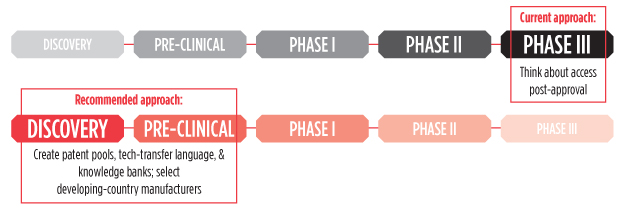
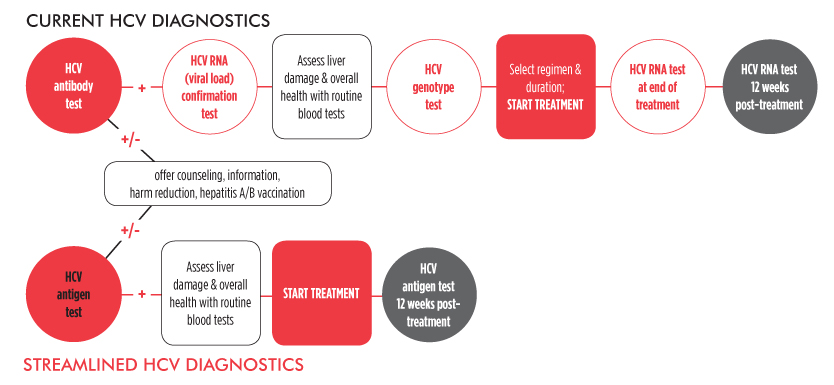
In Defense of Stringency
By Tim Horn In response to growing public concern with health risks posed by approved drugs, a 2006 landmark report by the Institute of Medicine (IOM) argued that the U.S. Food and Drug Administration (FDA) lacks the unambiguous authority necessary…