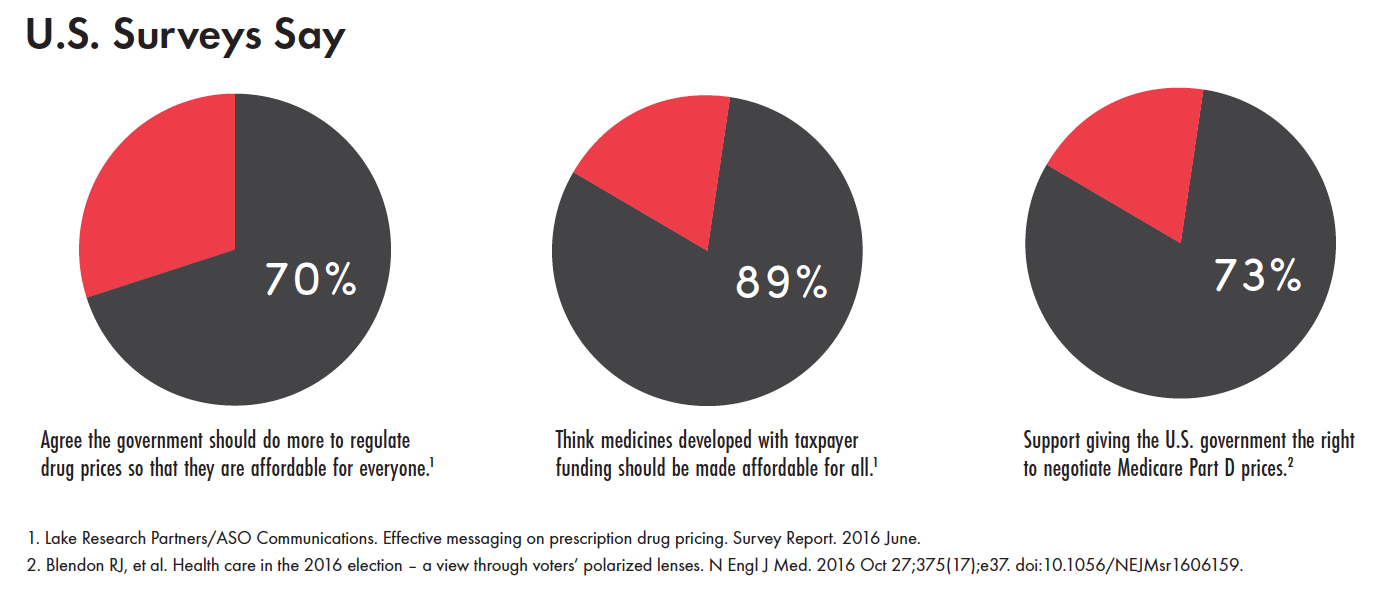
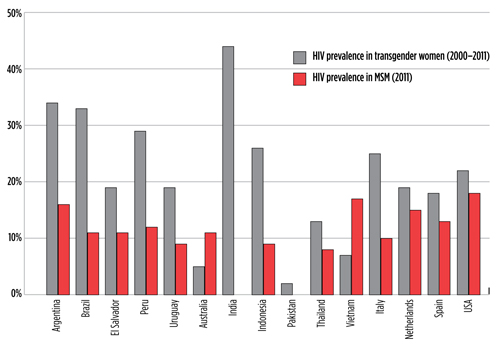
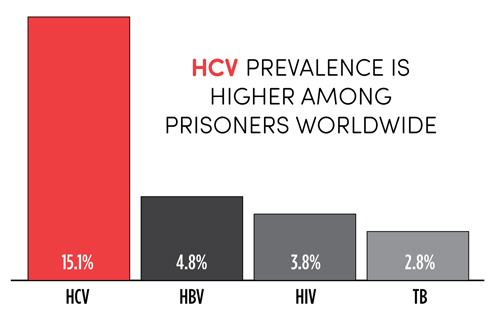
TAGline Spring 2017
With every major election, particularly one that secures or fortifies Republican control of the White House, Senate, or House of Representatives, a certain amount of worry and strategy realignment is to be expected from public health activists and civil society…