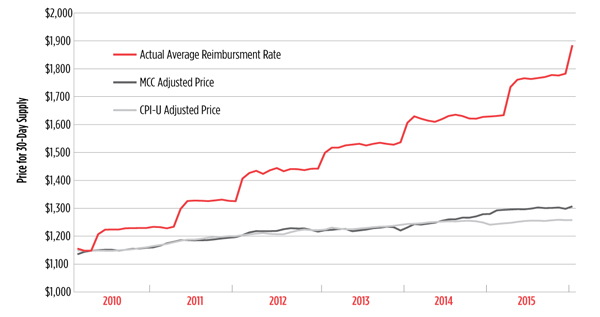
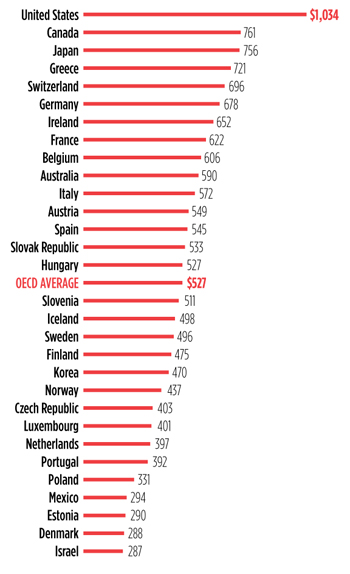
PrEP Pricing Problems
A number of barriers to pre-exposure prophylaxis (PrEP) uptake, use, and adherence have been identified—cost shouldn’t be one of them By James Krellenstein and Jeremiah Johnson On July 16, 2012, the U.S. Food and Drug Administration (FDA) approved Gilead Sciences’…